| Year |
Scientist |
Contribution |
Supporting Evidence |
|---|
1803
1808 |
John Dalton |
- Matter is composed of tiny indivisible particles called atoms.
- All atoms of the one element are identical but are different from the atoms of other elements.
- Chemical reactions consist of rearranging atoms in simple whole number ratios.
|
Law of Mass Conservation
(Lavoisier, 1789).
Law of Constant Composition
(Proust, 1799).
Law of Multiple Proportions
(Dalton).
|
| Year |
Scientist |
Contribution |
Supporting Evidence |
|---|
| 1904 |
Joseph J. Thomson |
"Plum Pudding" model of the divisible atom:
- Atoms consist of a large sphere of uniform positive charge embedded with smaller negatively charged particles (corpuscles).
- The total positive charge of the sphere equals the total negative charge of the corpuscles.
|
Experiments with cathode ray (Crookes) tubes showed that:
- 'canal rays' (positive charge) were different when different gases were used.
- 'cathode rays' (negative charge) were always identical regardless of the nature of the electrodes or gas used.
|
| Year |
Scientist |
Contribution |
Supporting Evidence |
|---|
| 1911 |
Ernest Rutherford |
Proposed a nuclear model of the atom in which:
- a very small positively charged nucleus containing most of the mass of the atom
- a very large volume around the nucleus in which electrons move
- a nucleus containing positively charged protons
- a number of protons equal to the number of electrons
He later postulated the existence of a neutral particle in the nucleus to make up for the calculated mass deficiency in the atoms studied.
|
Rutherford's 'gold foil' experiment performed by Hans Geiger and Ernest Marsden using positively charged alpha particles:
- Most alpha particles passed through the gold foil suggesting that an atom is largely empty space.
- Some alpha partcles were deflected significantly suggesting that the positive charge of an atom must be concentrated in a very small sphere.
- A very small number of alpha particles actually bounced back.
|
| Year |
Scientist |
Contribution |
Supporting Evidence |
|---|
| 1913 |
Niels Bohr |
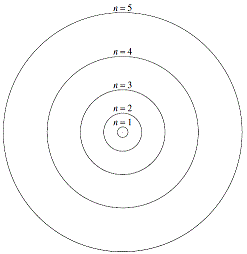
Proposed a 'planetary' model for the hydrogen atom (as shown on the left):
- Electrons move around the nucleus in fixed orbits (like planets around the sun).
An electron in a particular orbit has constant energy.
- An electron can absorb energy and move to a higher energy orbit of larger radius.
(excited electrons)
- An excited electron can fall back to its original orbit by emitting energy as radiation.
- Electrons can only exist in certain discrete energy levels.
|
Emission spectra of hydrogen:
- Lyman Series (ultraviolet):
corresponds to excited electrons falling back to the lowest energy level (smallest orbit) known as the ground state.
- Balmer Series (visible):
corresponds to excited electrons falling from higher energy levels to the first excited level.
- Paschen and Brackett Series (infrared):
excited electrons faling back to the 2nd, 3rd, 4th energy levels.
|
|
| Year |
Scientist |
Contribution |
Supporting Evidence |
|---|
| 1932 |
James Chadwick |
The nucleus of an atom contains neutrons, electrically neutral particles with a mass similar to that of a proton. |
Bombarded beryllium with alpha particles and discovered Rutherford's missing neutral particles.
The discovery of neutrons explained the existence of isotopes, first observed in 1920 by Francis Aston when he invented the mass spectrograph.
|
| Year |
Scientist |
Contribution |
Supporting Evidence |
|---|
| Current |
Quantum Mechanical Model of the Atom |
- Electrons occupy orbitals, volumes of space around the nucleus with a high probability of finding the electron.
- Energy levels are made up of energy sublevels.
- Each sublevel contains a set of orbitals.
- No orbital can contain more than 2 electrons (Pauli Exclusion Principle).
|
Schrödinger Equation for the hydrogen atom shows (1926):
- There are discrete energy levels.
- There is a significant probability of finding an electron of an atom at any position within a spherical volume surrounding the nucleus.
Wolfgang Pauli adapted the Schrödinger Equation for atoms containing more than 1 electron: the Pauli Exclusion Principle
|

