Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Naming Alkanoic Acids
Follow the steps below to name a straight-chain alkanoic acid
(also see tutorial Naming Straight-chain Alkanoic Acids):
- Number the longest carbon chain starting with the carbon atom of the COOH functional group.
| | H
| | | H
| | | H
| | | O
|| |
| H- | C4 | - | C3 | - | C2 | - | C1 |
| | |
H | | |
H | | |
H | | |
OH |
- Name the carbon chain as the alkane.
| Number of carbon atoms: | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|
| Prefix: | meth | eth | prop | but | pent | hex | hept | oct | non | dec |
|---|
alkane with 4 carbon atoms in the chain is named butane
- Drop the "e" from the name of the alkane.
butane becomes butan
- Add the suffix -oic acid
butan becomes butanoic acid
The preferred IUPAC names, formulae, and occurrence, of some alkanoic acids is given in the table below:
semi-structural formula
(molecular formula) |
longest carbon chain |
C-C
(single bonds) |
functional group |
Preferred IUPAC Name(4)
(alternative name) |
occurrence |
|---|
HCOOH
(CH2O2) |
C1
meth |
an |
-COOH (carboxyl)
oic acid |
formic acid
(methanoic acid) |
ant sting |
CH3COOH
(C2H4O2) |
C2
eth |
an |
-COOH (carboxyl)
oic acid |
acetic acid
(ethanoic acid) |
vinegar |
C2H5COOH
(C3H6O2) |
C3
prop |
an |
-COOH (carboxyl)
oic acid |
propanoic acid
(propionic acid) |
dairy products |
C3H7COOH
(C4H8O2) |
C4
but |
an |
-COOH (carboxyl)
oic acid |
butanoic acid
(butyric acid) |
rancid butter |
C4H9COOH
(C5H10O2) |
C5
pent |
an |
-COOH (carboxyl)
oic acid |
pentanoic acid
(valeric acid) |
valerian root |
Physical Properties of Alkanoic Acids
Alkanoic acids are weak acids, and as such are expected to have the properties of acids:
- taste sour
- conduct electricity in solution
- turn blue litmus red
Because alkanoic acids are capable of some dissociation in water, we expect them to display some solubility in water.
Alkanoic acids exist as covalent molecular substances, so their melting points and boiling points are expected to be quite low.
Melting Point and Boiling Point of Alkanoic Acids
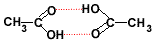 Alkanoic acids are polar molecules.
Alkanoic acids are polar molecules.
2 hydrogen bonds (shown in dotted red lines) can form between them.
For this reason, alkanoic acids have higher melting and boiling points than other compounds with the same number of carbon atoms, as shown in the table below:
Preferred IUPAC Name
(alternative name) |
Semi-structural Formula |
Number of atoms |
melting point
(°C) |
boiling point
(°C) |
|---|
| C atoms | O atoms |
|---|
acetic acid
(ethanoic acid) |
CH3COOH |
2 | 2 | more O |
17 | higher M.P. |
118 | higher B.P. |
| ethanol |
CH3CH2OH |
2 | 1 | ↑ |
-114 | ↑ |
78 | ↑ |
acetaldehyde
(ethanal) |
CH3CHO |
2 | 1 | ↑ |
-123 | ↑ |
20 | ↑ |
| ethane |
CH3CH3 |
2 | 0 | less O |
-183 | lower M.P. |
-89 | lower B.P. |
If two organic molecules have the same number of carbon atoms, then the molecule capabale of forming the greatest number of hydrogen-bonds will have the highest melting point and boiling point.
The melting point and the boiling points of alkanoic acids increase with increasing number of carbon atoms in the carbon chain.
The boiling points of alkanoic acids increase by about 20°C for each carbon that is added to the chain, as shown below:
Preferred IUPAC Name
(alternative name) |
Semi-structural Formula |
No. C atoms |
Molar Mass
(g mol-1) |
Trend |
Boiling Point (°C) |
|---|
formic acid
(methanoic acid) |
HCOOH |
1 |
46 |
lower |
100 |
acetic acid
(ethanoic acid) |
CH3COOH |
2 |
60 |
↓ |
118 |
| propanoic acid |
C2H5COOH |
3 |
74 |
↓ |
141 |
butanoic acid
(butyric acid) |
C3H7COOH |
4 |
88 |
↓ |
164 |
| pentanoic acid |
C4H9COOH |
5 |
102 |
↓ |
186 |
| hexanoic acid |
C5H11COOH |
6 |
116 |
↓ |
205 |
| heptanoic acid |
C6H13COOH |
7 |
130 |
higher |
223 |
The trend is even more obvious if we graph boiling points of this series of alkanoic acids as shown below:
| Boiling Point (oC)
|
Boiling Point of Alkanoic (Carboxylic) Acids
Molar Mass (g mol-1)
|
As the carbon chain becomes longer, the intermolecular interactions between these carbon chains becomes greater, so the weaker Van der Waals forces (dispersion forces or london forces) (5) become more significant, and the amount of energy required to separate the molecules so that the liquid will boil becomes greater.
Solubility of Alkanoic Acids in Water
The table below gives the solubility in water of a number of alkanoic acids.
Can you see a pattern, or trend, in the data?
Preferred IUPAC Name
(alternative name) |
Semi-structural Formula |
No. C atoms |
Molar Mass
(g mol-1) |
Solubility
(g/100g water) |
Trend |
|---|
formic acid
(methanoic acid) |
HCOOH |
1 |
46 |
miscible |
|
acetic acid
(ethanoic acid) |
CH3COOH |
2 |
60 |
miscible |
|
| propanoic acid |
C2H5COOH |
3 |
74 |
miscible |
|
butanoic acid
(butyric acid) |
C3H7COOH |
4 |
88 |
miscible |
|
| pentanoic acid |
C4H9COOH |
5 |
102 |
3.4 |
greater |
| hexanoic acid |
C5H11COOH |
6 |
116 |
1.0 |
↓ |
| heptanoic acid |
C6H13COOH |
7 |
130 |
0.2 |
least |
Short chain alkanoic acids, that is formic acid (methanoic acid) to butanoic acid (butyric acid), are soluble (miscible) in water as a result of hydrogen bonding between the OH group of the carboxyl group and water molecules.
However, after butanoic acid, as the number of carbon atoms in the carbon chain increases, the solubility of the alkanoic acid in water decreases.
The weak intermolecular forces of attraction between the hydrocarbon chains (Dispersion forces or London forces) become more significant as the chains become longer, so the alkanoic acids are more attracted to each other than they are to the polar water molecules.
Chemical Properties of Alkanoic Acids
Alkanoic acids are weak acids, the acid dissociation constant, Ka, is small.
Soluble alkanoic acids dissociate slightly in water :
| general reaction: | R-COOH | ⇋ | R-COO-(aq) | + | H+(aq) |
relative amount of species
present in aqueous solution: | almost 100% | | small amount | | small amount |
The table below gives that value of the acid dissociation constant, Ka, for acetic acid and chlorinated acetic acid derivatives.
Can you see a pattern, or a trend, in the data?
Preferred IUPAC Name
(alternative name) |
Semi-structural Formula |
Number of chlorine atoms |
Acid Dissociation Constant
(Ka) |
acidity |
|---|
acetic acid
(ethanoic acid) |
CH3COOH |
0 |
10-4.8 |
lower |
chloroacetic acid
(chloroethanoic acid) |
ClCH2COOH |
1 |
10-2.9 |
↓ |
dichloroacetic acid
(dicholorethanoic acid) |
Cl2CHCOOH |
2 |
10-1.3 |
↓ |
trichloroacetic acid
(trichloroethanoic acid) |
Cl3CCOOH |
3 |
10-0.7 |
higher |
As the number of chlorine atoms substituting for hydrogen in the acetic acid molecule increase, the value of the acid dissociation constant also increases.
The acidity of carboxylic acids increases with the substitution of highly electronegative atoms, such as chlorine, in the molecule.
Alkanoic acids react with:
Alkanoic Acids and Neutralisation Reactions
Alkanoic acids are acids, they react with bases to produce a salt and water in a neutralisation reaction.
| neutralisation reaction: | acid | + | strong base | → | salt | + | water |
| general word equation: | carboxylic acid
(alkanoic acid) | + | strong base | → | salt
(metal alkanoate) | + | water |
| general chemical equation: | RCOOH | + | MOH | → | RCOO-M+ | + | H2O |
| chemical equation example: | CH3COOH | + | NaOH | → | CH3COO-Na+ | + | H2O |
| word equation example: | acetic acid
(ethanoic acid) | + | sodium hydroxide | → | sodium acetate
(sodium ethanoate) | + | water |
The salt produced (metal alkanoate) is the salt of a strong base and weak acid, so the aqueous salt solution produced in the neutralisation reaction will be basic (pH > 7 at 25°C)
Soluble salts of long-chain carboxylic acids (fatty acids) are soaps
| neutralisation reaction: | acid | + | strong base | → | salt | + | water |
| general word equation: | long-chain carboxylic acid
(fatty acid) | + | strong base | → | soap
(soluble metal alkanoate) | + | water |
| general chemical equation: | RCOOH | + | MOH | → | RCOO-M+ | + | H2O |
| chemical equation example: | C17H35COOH | + | NaOH | → | C17H35COO-Na+ | + | H2O |
| word equation example: | stearic acid | + | sodium hydroxide | → | sodium stearate | + | water |
Alkanoic Acids React with Carbonates
Alkanoic acids are acids, they react with carbonates (including hydrogen carbonates) to produce a salt, carbon dioxide gas and water.
| general reaction: | acid | + | carbonate | → | salt | + | carbon dioxide gas | + | water |
| general word equation: | alkanoic acid | + | metal carbonate | → | metal alkanoate | + | carbon dioxide | + | water |
| general chemical equation: | RCOOH | + | M2CO3 | → | 2RCOO-M+ | + | CO2(g) | + | H2O |
| chemical equation example: | 2CH3COOH | + | Na2CO3 | → | 2CH3COO-Na+ | + | CO2(g) | + | H2O |
| word equation example: | acetic acid
(ethanoic acid) | + | sodium carbonate | → | sodium acetate
(sodium ethanoate) | + | carbon dioxide | + | water |
|
| chemical equation example: | CH3COOH | + | NaHCO3 | → | CH3COO-Na+ | + | CO2(g) | + | H2O |
| word equation example: | acetic acid
(ethanoic acid) | + | sodium hydrogen carbonate (6)
(sodium bicarbonate) | → | sodium acetate
(sodium ethanoate) | + | carbon dioxide | + | water |
The standard test for carbon dioxide gas is to bubble the gas through limewater to produce a "milky" precipitate.
Alkanoic Acids React with Active Metals
Alkanoic acids are acids, they react with active metals such as Group 1 (IA or alkali metal) elements to produce a salt and hydrogen gas.
| general reaction: | acid | + | active metal | → | salt | + | hydrogen gas |
| general word equation: | alkanoic acid | + | active metal | → | metal alkanoate | + | hydrogen gas |
| general chemical equation: | 2RCOOH | + | 2M(s) | → | 2RCOO-M+ | + | H2(g) |
| chemical equation example: | 2CH3COOH | + | 2Na(s) | → | 2CH3COO-Na+ | + | H2(g) |
| word reaction example: | acetic acid
(ethanoic acid) | + | sodium | → | sodium acetate
(sodium ethanoate) | + | hydrogen gas |
The standard test for hrogen gas is the "pop test".
Esterification Reaction
Esters are produced in a condensation reaction between an alkanoic acid (carboxylic acid) and an alkanol (alcohol).
A condensation reaction is a special type of elimination reaction in which water is the small molecule being eliminated during the reaction.
The reaction that produces an ester is known as an esterification reaction.
| general word equation: | carboxylic acid
(alkanoic acid) | + | alcohol
(alkanol) | ⇋ | ester | + | water |
| general chemical equation: | RCOOH | + | R'OH | ⇋ | RCOOR' | + | H2O |
| chemical equation example: | CH3COOH | + | CH3OH | ⇋ | CH3COOCH3 | + | H2O |
| word reaction example: | acetic acid
(ethanoic acid) | + | methanol
(methyl alcohol) | ⇋ | methyl acetate
(methyl ethanoate) | + | water |
Esters commonly have fragrent, fruity odours. You can use your sense of smell to detect the synthesis of an ester.
(1) Alkanoic acids are those carboxylic acids in which an oxygen atom (=O) has been substituted for two of the hydrogen atoms in the corresponding alkane, and, an OH functional group has substituted for another H atom on the same carbon atom.
(2) This refers to the naming of carboxylic acids in which there is only one functional group, the COOH functional group, and that occurs on a terminal carbon atom of an alkane chain.
(3) The term neutralisation reactions is not the best description of the reaction between a weak acid, such as a carboxylic acid, and a base, because the resulting solution at the equivalence point is not neutral. Instead, they are better described as proton transfer reactions.
(4) The rules for naming organic compounds are still being developed. The most recent document for referral is "Preferred names in the nomenclature of organic compounds" (Draft 7 October 2004).
(5) Some Chemists refer to all intermolecular forces as Van der Waal's forces, others use the term Van der Waal's forces synonymously with London forces or dispersion forces. It is probably best to avoid using the term Van der Waal's forces at all and use one of the other, unambiguous, terms instead.
(6) If you are an organic chemist, you name the HO-CO-O- ion as the hydrogen carbonate ion (two words separated by a space, hydrogen carbonate).
If you are an inorganic chemist, you name the same ion as the hydrogencarbonate ion (one word, hydrogencarbonate).
