Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Types of Intermolecular Forces
Three types of force can operate between covalent molecules:
- Dispersion Forces: also known as
⚛ London Forces (named after Fritz London who first described these forces theoretically 1930)
⚛ Weak Intermolecular Forces
⚛ van der Waal's Forces2 (namd after the person who contributed to our understanding of non-ideal gas behaviour).
- Dipole-dipole interactions
- Hydrogen bonds
1. Dispersion Forces (London Forces, Weak Intermolecular Forces, van der Waal's Forces):
- Dispersion forces are very weak forces of attraction that act between all molecules and result from:
(i) momentary dipoles occurring due to uneven electron distributions in neighbouring molecules as they approach one another
(ii) the weak residual attraction of the nuclei in one molecule for the electrons in a neighbouring molecule.
- The more electrons that are present in the molecule, the stronger the dispersion forces will be.
- Dispersion forces are the only type of intermolecular force operating between non-polar molecules, for example, dispersion forces operate between:
⚛ hydrogen (H2) molecules in a volume of hydrogen gas
⚛ chlorine (Cl2) molecules in a volume of chlorine gas
⚛ carbon dioxide (CO2) molecules in a volume of carbon dioxide gas
⚛ dinitrogen tetroxide (N2O4) molecules in a volume of dinitrogen tetroxide gas
⚛ methane (CH4) molecules in a volume of methane gas
2. Dipole-dipole Interactions
3. Hydrogen Bonds
- Hydrogen bonds occur between molecules that have a permanent net dipole resulting from hydrogen being covalently bonded to either fluorine, oxygen or nitrogen.
For example, hydrogen bonds operate between:
⚛ water (H2O) molecules in a volume of liquid water
⚛ ammonia (NH3) molecules in a volume of ammonia gas
⚛ hydrogen fluoride (HF) molecules in a volume of hydrogen fluoride gas
⚛ hydrogen peroxide (H2O2) molecules in a volume of hydrogen peroxide
⚛ alkanols (alcohols) such as methanol (CH3OH) molecules in a volume of liquid methanol
⚛ alkanoic acids (caboxylic acids) such as acetic acid (ethanoic acid, CH3COOH) in a volume of liquid acetic acid
⚛ organic amines such as methanamine (methyl amine, CH3NH2) in a volume of methanamine
- Hydrogen bonds are a stronger intermolecular force than either Dispersion forces or dipole-dipole interactions since the hydrogen nucleus is extremely small and positively charged and fluorine, oxygen and nitrogen being very electronegative so that the electron on the hydrogen atom is strongly attracted to the fluorine, oxygen or nitrogen atom, leaving a highly localised positive charge on the hydrogen atom and highly negative localised charge on the fluorine, oxygen or nitrogen atom. This means the electrostatic attraction between these molecules will be greater than for the polar molecules that do not have hydrogen covalently bonded to either fluorine, oxygen or nitrogen.
Effect of Intermolecular forces on Melting Points and Boiling Points of Molecular Covalent Substances
Since melting or boiling result from a progressive weakening of the attractive forces between the covalent molecules, the stronger the intermolecular force is, the more energy is required to melt the solid or boil the liquid.
If only dispersion forces are present, then the more electrons the molecule has (and consequently the more mass it has) the stronger the dispersion forces will be, so the higher the melting and boiling points will be.
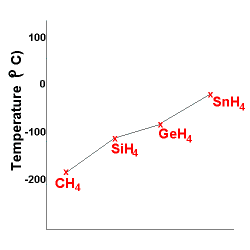
Consider the hydrides of Group 14 elements, all of which are non-polar molecules, so only dispersion forces act between the molecules.
The Group 14 hydrides are:
CH4 (molecular mass ≈ 16)
SiH4 (molecular mass ≈ 32)
GeH4 (molecular mass ≈ 77)
SnH4 (molecular mass ≈ 123)
All of these molecules can all be considered non-polar covalent molecules.
As the mass of the molecules increases, so does the strength of the dispersion force acting between the molecules.
As the strength of the dispersion forces acting between the molecules increases, more energy is required to weaken the attraction between the molecules resulting in higher boiling points.
So, as the mass of the Group 14 hydride molecules increase, the boiling point of the substance increases as shown by the graph below:
If a covalent molecule has a permanent net dipole then the force of attraction between these molecules will be stronger than if only dispersion forces were present between the molecules.
As a consequence, this substance will have a higher melting point or boiling point than similar molecules that are non-polar in nature.
Consider the boiling points of the hydrides of Group 17 (halogen) elements.
The hydrides of Group 17 (halogen) elements are:
HF (molecular mass ≈ 20)
HCl (molecular mass ≈ 37)
HBr (molecular mass ≈ 81)
HI (molecular mass ≈ 128)
All of these molecules are polar, the hydrogen atom having a partial positive charge (Hδ+) and the halogen atom having a partial negative charge (Fδ-, Clδ-, Brδ-, Iδ-).
As a consequence, the stronger dipole-interactions acting between the hydride molecules of Group 17 elements results in higher boiling points than for the hydrides of Group 14 elements as seen above.
The boiling point of the hydrides of Group 17 elements is shown in the graph below:
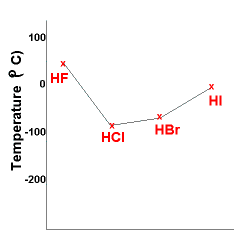
With the exception of HF, as the molecular mass increases, the boiling point of the hydrides increase.
HF is an exception because of the stronger force of attraction between HF molecules resulting from hydrogen bonds acting bewteen the HF molecules.
Weaker dipole-dipole interactions act between the molecules of HCl, HBr and HI.
So HF has a higher boiling point than the other molecules in this series.
There is a full discussion of the effect of intermolecular forces on the group 16 hydrides in the Trends in Group 16 Hydrides tutorial.


