Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Physical Properties of Ionic Compounds: High Melting Point
Ionic compounds have high melting points.
The electrostatic attraction (ionic bond) between cations and anions is strong.
It takes a lot of energy to overcome this attraction in order to allow the ions to move more freely and form a liquid.
The factors which affect the melting point of an ionic compound are:
- The charge on the ions.
- Size of the ions.
(i) Charge on the Ions
In general, the greater the charge, the greater the electrostatic attraction, the stronger the ionic bond, the higher the melting point.
The table below compares the melting point and ion charges for two ionic compounds, sodium chloride (NaCl) and magnesium oxide (MgO).
| Ionic Compound |
Melting Point (°C) |
Cation Charge |
Anion Charge |
|---|
NaCl
(Na+Cl-) |
801 |
+1 |
-1 |
MgO
(Mg2+O2-) |
2800 |
+2 |
-2 |
MgO has a higher melting point than NaCl because 2 electrons are transferred from magnesium to oxygen to form MgO while only 1 electron is transferred from sodium to chlorine to form NaCl.
(ii) The size of the ions.
Smaller ions can pack closer together than larger ions so the electrostatic attraction is greater, the ionic bond is stronger, the melting point is higher.
The melting point of Group 1 (alkali) metal fluorides is compared to the ionic radius of the cation in the table below.
| Ionic Compound |
Melting Point (°C) |
Cation Radius (pm) |
|---|
| NaF |
992 |
higher M.P. |
99 |
smaller radius |
| KF |
857 |
↑ |
136 |
↓ |
| RbF |
775 |
↑ |
148 |
↓ |
| CsF |
683 |
lower M.P. |
169 |
larger radius |
As the radius of the cations increases down Group 1 from Na+ to Cs+, the melting points of the fluorides decrease.
Physical Properties of Ionic Compounds: Conductivity
In order for a substance to conduct electricity it must contain mobile particles capable of carrying charge.
| |
Ionic Solid |
Ionic Liquid |
Aqueous Solution |
|---|
| Mobility of Ions |
very poor |
good |
good |
|---|
| Electrical Conductivity |
very poor |
good |
good |
|---|
Solid ionic compounds do not conduct electricity because the ions (charged particles) are locked into a rigid lattice or array.
The ions cannot move out of the lattice, so the solid cannot conduct electricity.
When heated, the ionic solid melts to form a liquid, or a molten, ionic compound.
The ions in the molten, or liquid, ionic compound are free to move out of the lattice structure.
When an electric current is passed through a molten ionic compound:
- Cations (positive ions) move towards the cathode
M+(l) + e- → M(l)
- Anions (negative ions) move towards the anode
X-(l) → X + e-
When an ionic solid is dissolved in water to form an aqueous solution:
MX(aq) → M+(aq) + X-(aq)
the ions are released from the lattice structure and are free to move so the solution conducts electricity just like the molten (liquid) ionic compound.
Physical Properties of Ionic Compounds: Brittleness
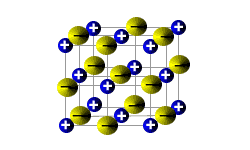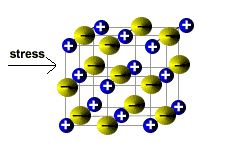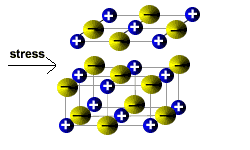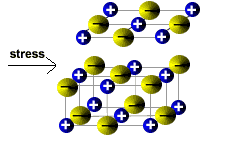
Ionic solids are brittle.
When a stress is applied to the ionic lattice, the layers shift slightly.
The layers are arranged so that each cation is surrounded by anions in the lattice.
If the layers shift then ions of the same charge will be brought closer together.
Ions of the same charge will repel each other, so the lattice structure breaks down into smaller pieces.