Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
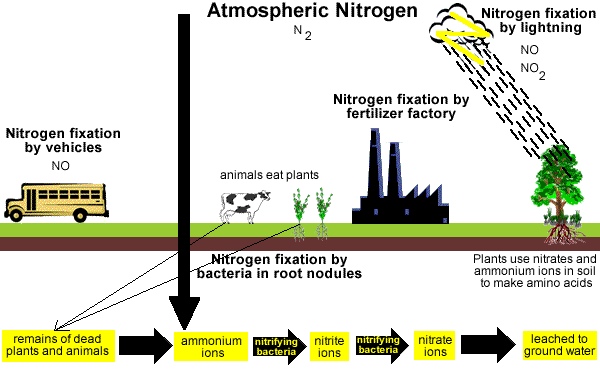
Nitrogen Cycle
N2 gas in the atmosphere can be changed into soluble nitrogen compounds in the soil.
Electrical storms can produce small quantities of nitrates (NO3-) and nitrates (NO2-) which are carried to earth in rain.
Some plants, like legumes, can convert atmospheric N2 gas into nitrogen compounds which can be converted by bacteria into nitrates (NO3-), which plants can use to make plant proteins.
Soluble nitrogen compounds in soil can be taken up by plants to make plant proteins.
Animals eat the plants and by the process of digestion convert the plant proteins to animal proteins.
Plants and animals die and their decomposing cells release N2 gas back into the atmosphere.
The nitrogen cycle involves oxidation and reduction of nitrogen.