Reducing and Non-reducing Sugars Chemistry Tutorial
Key Concepts
Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Structure and Classification of Monosaccharides: Aldoses and Ketoses
We can classify a monosaccharide on the basis of its open ring or chain structure.
This structural classification of the open-ring, or chain, form of a monosaccharide depends on:
- location of the carbonyl (C=O) functional group
- the number of carbon atoms in the chain
When the closed-ring structure (cyclic structure) of a monosaccharide opens to form a chain, the result may be either
- an aldehyde (terminal carbonyl, C=O, functional group)
- a ketone (non-terminal carbonyl, C=O, functional group)
Monosaccharides that are aldehydes are known as aldoses.
("ald" from aldehyde + "ose" for a reducing sugar.)
Sugars that are ketones are known as ketoses.
("ket" from ketone + "ose" for a reducing sugar.)
an aldehyde
Sugars that are aldehydes are known as aldoses.
Examples: glucose, galactose, ribose, deoxyribose
|
|
a ketone
Sugars that are ketones are known as ketoses.
Example: fructose
|
There are usually 3 to 7 carbon atoms in a monosaccharide.
The number of carbon atoms is indicated by the prefixes:
- tri (3)
- tetr (4)
- pent (5)
- hex (6)
- hept (7)
For example, a monosaccharide containing 5 carbon atoms is known as a pentose.
"pent" for 5 + "ose" for a reducing sugar.
| Number of Carbon atoms: |
3 |
4 |
5 |
6 |
7 |
|---|
| Classification of Monosaccharide: |
triose |
tetrose |
pentose |
hexose |
heptose |
|---|
The location of the carbonyl carbon (C=O) then decides if it is an aldehyde with the "ald" prefix or a ketone with the "ket" prefix.
For example, the open-chain form of a pentose could be either:
- an aldopentose if it is an aldehyde
- or a ketopentose if it is a ketone
A monosaccharide containing 6 carbon atoms is known as a hexose.
The open-chain form could be either:
- an aldohexose if it is an aldehyde (alkanal)
- or a ketohexose if it is a ketone (alkanone)
The table below gives an overview of how we classify monosaccharides:
Number of
Carbon atoms: |
Classification of Monosaccharides |
|---|
| General |
of Aldehydes |
of Ketones |
| 3 |
triose |
aldotriose |
ketotriose |
| 4 |
tetrose |
aldotetrose |
ketotetrose |
| 5 |
pentose |
aldopentose |
ketopentose |
| 6 |
hexose |
aldohexose |
ketohexose |
| 7 |
heptose |
aldoheptose |
ketoheptose |
Glucose and fructose are both monosaccharides with 6 carbon atoms in the open-ring, or chain, form of the structure.
Glucose and fructose are therefore both classified as hexoses.
However, the chain structure of glucose contains a terminal carbonyl (C=O) functional group which makes it an aldohexose.
Fructuose, on the other hand, has a non-terminal carbonyl (C=O) functional group which makes it a ketohexose.
Compare the closed-ring and open-ring (or chain) structures of glucose and fructose as shown in the table below:
| Hexose |
Glucose |
Fructose |
|---|
| Closed-ring (cyclic) Structure |
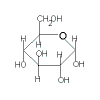 |
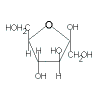 |
|---|
| Open-chain Structure |
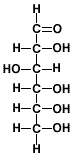 |
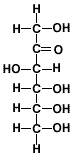 |
|---|
| Classification |
aldohexose |
ketohexose |
|---|
Testing for the Presence of a Reducing Sugar
Another to classify sugars is on the basis of a chemical reaction, that is, on whether the sugar can be oxidised or not.
If a carbonyl group can undergo mild oxidation it will produce a carboxylate ion (COO-).
A sugar that can be oxidised is known as a reducing sugar.
A reducing agent is a species that causes a different species to be reduced.
In the process, the reducing agent is itself oxidised.
Therefore a sugar that is oxidised must be causing another species to be reduced, so the sugar is a reducing agent (or reductant), or a reducing sugar.
A sugar that can NOT be oxidised is known as a non-reducing sugar.
In order to test whether a sugar can be oxidised or not, we need to add a species that can undergo reduction.
A species that undergoes reduction is known as an oxidising agent, or oxidant, because it causes the other species (the sugar) to be oxidised.
Blue solutions of copper ions, Cu2+, are popular oxidising agents for sugars because they produce an insoluble Cu2O precipitate which is a brick-red colour that is easy to see.
The oxidation state of Cu in Cu2+ is +2
The oxidation state of Cu in Cu2O is +1
Since the oxidation state of Cu has decreased, the copper "atoms" have gained an electron, that is the copper "atoms" have been reduced.
Cu2+ is therefore an oxidising agent (also known as an oxidant).
Cu2+ is found in both Benedict's solution and in Fehling's solution which are two solutions that are used to test for the presence of a reducing sugar.
Another solution that is commonly used to test for a reducing a sugar is Tollen's reagent.
Tollen's reagent does not contain Cu2+, instead it contains colourless silver ions, Ag+.
When silver ions gain an electron (are reduced), the result is solid silver:
The oxidation state of Ag in Ag+ is +1
The oxidation state of Ag in Ag(s) is 0
The oxidation state of Ag has decreased from +1 to 0, Ag+ has gained an electron, so Ag+ has been reduced.
Ag+ is the oxidising agent, or oxidant.
The solid silver forms a visible "mirror" which makes it easy to determine whether the sugar has been oxidised.
The table below compares these three solutions commonly used to test for the presence of a reducing sugar: Benedict's solution, Fehling's solution and Tollen's reagent:
| Oxidising Reagent |
Benedict's Solution |
Fehling's Solution |
Tollen's Reagent |
|---|
| Composition |
copper sulfate in alkaline citrate |
copper sulfate in alkaline tartrate |
silver nitrate in aqueous ammonia |
|---|
| Colour of Solution |
deep blue |
deep blue |
colourless |
|---|
| Colour After Reaction with a Reducing Sugar |
brick red precipitate
Cu2O(s) |
brick red precipitate
Cu2O(s) |
silver mirror forms
Ag(s) |
|---|
Species Being Reduced
(oxidising agent) |
Cu2+ |
Cu2+ |
Ag+ |
|---|
| Reduction Reaction |
Cu2+ + e- → Cu+ |
Cu2+ + e- → Cu+ |
Ag+ + e- → Ag(s) |
|---|
Species Being Oxidised
(reducing agent) |
reducing sugar is oxidised to carboxylate |
reducing sugar is oxidised to carboxylate |
reducing sugar is oxidised to carboxylate |
|---|
In general, an aldehyde functional group is easily oxidised to a carboxyl functional group.
We expect aldose sugars to be reducing sugars.
If one of the mild oxidising reagents above is added to a monosaccharide aldose sugar you expect to see the relevant colour change.
Although fructose is a ketose sugar containing the ketone functional group, it is also a reducing sugar.
Of the disaccharides, maltose and lactose are reducing sugars, but sucrose is NOT.
Sucrose is a non-reducing sugar.
If you add one of the mild oxidising agents above to sucrose, there will be NO reaction, no colour change.
Reducing Sugars: Mild Oxidation of Monosaccharides
The carbonyl group (C=O) in an aldose is readily oxidised to a carboxylate group
Glucose and galactose are both examples of aldose sugars.
Glucose and galactose can be oxidised by a mild oxidising agent.
Consider the reaction between an aldose such as glucose or galactose and Benedict's solution (or Fehling's solution) as shown below:
| aldose |
|
carboxylate |
|
|
|
+ 2Cu2+ + 5OH- |
⇋ |
|
+ Cu2O(s) |
+ 3H2O |
| |
deep blue |
|
brick red |
|
The aldehyde C=O group has been oxidised to a carboxylate ion (COO-).
The copper has been reduced.
You will observe a brick-red precipitate.
You will conclude that the aldose is a reducing sugar.
Now consider the reaction between an aldose and Tollen's Reagent as shown below:
| aldose |
|
|
carboxylate |
|
|
|
|
+ 2Ag(NH3)2+ + 3OH- |
⇋ |
|
+ 2Ag(s) |
+ 4NH3(g) + 2H2O |
| |
colourless |
|
|
silver mirror |
|
The aldehyde C=O group has been oxidised to a carboxylate ion (COO-).
The silver ions have been reduced.
You will observe the precipitation of solid silver as a silver mirror.
You will conclude that the aldose is a reducing sugar.
Although the carbonyl group of a ketone is usually not easily oxidised, there are exceptions.
For example, fructose is an example of a ketose that is a reducing sugar.
Fructose can be oxidised by a mild oxidising agent.
The product of the oxidation reaction is a hydroxy carboxylate.
The products of the oxidation reaction contains both the hydroxyl (OH) group and the carboxylate (COO-) group.
Consider the reaction between fructose and Benedict's solution or Fehling's solution as shown below:
| ketose |
|
|
hydroxy carboxylate |
|
|
|
|
+ 2Cu2+ + 5OH- |
⇋ |
|
+ Cu2O(s) |
+ 3H2O |
| |
deep blue |
|
|
brick red |
|
The copper has been reduced.
You will observe a brick-red precipitate.
You will conclude that this ketose, fructose, is a reducing sugar.
Now consider the reaction between Tollen's reagent and fructose as shown below:
| ketose |
|
|
hydroxy carboxylate |
|
|
|
|
+ 2Ag(NH3)2+ + 3OH- |
⇋ |
|
+ 2Ag(s) |
+ 4NH3(g) + 2H2O |
| |
colourless |
|
|
silver mirror |
|
Silver ions have been reduced.
You will observe a "silver mirror" effect.
You will conclude that this ketose, fructose, is a reducing sugar.
Structure and Mild Oxidation of Disaccharides
Some disaccharides are reducing sugars, they can be oxidised by mild oxidising agents.
Examples are maltose and lactose.
Some disaccharides are non-reducing sugars, they can NOT be oxidised by mild oxidising agents.
Sucrose is an example of a non-reducing sugar.
The table below gives the key structural features of reducing and non-reducing disaccharides as well as the results of reactions with mild oxidising agents like Benedict's solution, Fehling's solution and Tollen's reagent:
| Disaccharide |
sucrose |
maltose |
lactose |
|---|
| Cyclic Structure |
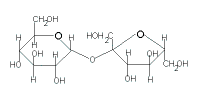 |
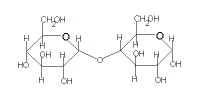 |
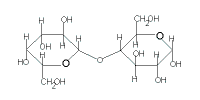 |
|---|
| Glycosidic Link through |
2 carbonyl carbons |
1 carbonyl carbon |
1 carbonyl carbon |
|---|
| Open-chain structure |
no |
yes |
yes |
|---|
| +Benedict's Solution |
no reaction |
forms brick red solid |
forms brick red solid |
|---|
| +Fehling's Solution |
no reaction |
forms brick red solid |
forms brick red solid |
|---|
| +Tollen's Reagent |
no reaction |
forms silver mirror |
forms silver mirror |
|---|
| Classification |
non-reducing sugar |
reducing sugar |
reducing sugar |
|---|
Mild Oxidation of Polysaccharides
Polysaccharides such as starch and cellulose can not be oxidised using mild oxidising agents like Benedict's solution, Fehling's solution or Tollen's reagent.
If you test a polysaccharide with each of these reagents there will be no reaction.
| Mild Oxidising Agent |
Reaction with Polysaccharide |
|---|
| Benedict's solution |
no colour change
(stays blue) |
|---|
| Fehling's solution |
no colour change
(stays blue) |
|---|
| Tollen's reagent |
no colour change
(stays colourless) |
|---|
Worked Example of a Reducing Sugars Problem
Problem Solving using the StoPGoPS approach.
Question: Glucose is also known as blood sugar because it is present in a our bloodstream.
Our kidneys filter out the glucose so that there should be no glucose present in our urine.
A person who suffers from diabetes will have glucose present in their urine.
Chris the Chemist has been given a sample of a patient's urine and asked to determine if the patient is suffering from diabetes.
Chris safely adds a few drops of Benedict's solution to a small sample of the urine.
The results are shown below:
| |
Colour of urine sample |
|---|
| before addition of Benedict's Solution |
pale yellow |
| after addition of Benedict's Solution |
small amount of red precipitate |
Will Chris report that it is possible that the patient has diabetes or not?
|
| STOP |
STOP! State the Question.
|
| |
What is the question asking you to do?
Determine whether the patient could have diabetes.
|
| PAUSE |
PAUSE to Prepare a Game Plan
|
| |
(1) What information (data) have you been given in the question?
(a) Glucose is present in the urine of a diabetic.
(b) Benedict's solution changed colour when added to the urine sample.
(2) What is the relationship between what you know and what you need to find out?
Glucose is a reducing sugar.
Benedict's solution will produce a brick-red precipitate of Cu2O in the presence of a reducing sugar.
If a reducing sugar is not present, the Benedict's solution will not change colour and will remain blue.
|
| GO |
GO with the Game Plan |
| |
| urine |
+ |
Benedict's solution |
→ |
brick red precipitate |
| pale yellow |
|
|
|
brick red precipitate |
Benedict's solution produced a brick-red precipitate which indicates the presence of a reducing agent such as a reducing sugar which could be glucose.
|
| PAUSE |
PAUSE to Ponder Plausibility |
| |
Is your answer plausible?
Work backwards: assume the patient has diabetes.
If the patient has diabetes, glucose will be present in the urine.
Glucose is a reducing sugar, that is it causes another reagent to be reduced.
Copper sulfate solutions are typically blue due to the presence of Cu2+(aq).
Benedict's solution contains Cu2+.
Glucose will cause Cu2+ to be reduced to Cu+, with the formation of insoluble Cu2O.
Benedict's solution will produce a precipitate if the urine contains glucose.
We have confirmed our solution that the urine sample could contain glucose and the patient may have diabetes.
|
| STOP |
STOP! State the Solution |
| |
It is possible that the patient has diabetes.
|
Footnotes:
(1) Procedure for making Benedict's solution:
Solution A: Measure out about 350 mL of water.
Add 86.5 g of crystallised sodium citrate and 50 g of anhydrous sodium carbonate to the water.
Stir to dissolve as much of the solids as possible.
Filter the solution.
Solution B: Make a solution of copper sulfate by dissolving 8.65 g of crystallised copper sulfate to 50 mL of water, stirring constantly.
Benedict's Solution: Pour both solutions (solution A and solution B) into a 500 mL volumetric flask and make up to the mark with water. Label this solution Benedict's solution.
The Benedict's solution should be clear. If it looks cloudy, filter it.
(2) Procedure for making Fehling's solution:
Solution A: dissolve 34.64 g of crystallised copper sulfate in water containing a few drops of dilute sulfuric acid in a 500 mL volumetric flask and make up to the mark with water.
Solution B: dissolve 60 g of pure sodium hydroxide and 173 g of pure sodium potassium tartrate in water. Filter if necessary. Dilute the solution to 500 mL with water.
Keep each of the two solutions in separate tightly stoppered vessels.
When you want to use them, combine equal volumes of each solution and mix.
(3) Procedure for making Tollen's reagent:
Solution A: Dissolve 3 g of silver nitrate in 30 mL of water.
Solution B: Dissolve 3 g of sodium hydroxide in 30 mL of water.
When you need to use the reagent, mix 1 mL of each solution (A and B) together in a test tube and add dilute ammonia solution drop by drop until the silver oxide is just dissolved.
To clean the test tube, rinse with dilute nitric acid.







