Safety in the Laboratory Chemistry Tutorial
Key Concepts
Safety in the Laboratory is the reponsibility of everyone in the laboratory.
This includes teachers, students, lab technicians (assistants) and visitors to the lab.
Before undertaking any practical work (experiments) in the laboratory, you should ask yourself the following 5 questions:
Question 1: Do I know the safety rules for working in this laboratory?
Question 2: Do I know where the safety equipment is located in this laboratory?
Question 3: What are the possible hazards (dangers) during this experiment?
Question 4: What are the possible consequences (risks) of these hazards?
Question 5: For this experiment, is it possible to:
(i) eliminate the risk?
Is it necessary to use this chemical?
Is it necessary to undertake this dangerous process?
(ii) substitute a safer alternative?
Can you use a different, less hazardous chemical?
Can you use a different, less hazardous process?
(iii) guard against the risk?
Will using particular safety equipment minimise the hazard?
If you are performing an experiment using the materials and method provided by your teacher, you will need to concentrate on thinking about how to guard against the possible risks.
If you are designing and carrying out your own experiments, you will need to confer with your teacher during the design process in order to determine whether the potential risks can be eliminated, substituted, and/or guarded against.
Download a free Student Safety Agreement.
Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Safety Rules
Different laboratories will have different safety rules depending on the type of experiments conducted in them.
Below is a list of general rules that would apply to most chemistry laboratories.
| Safety Rules for a Chemistry Laboratory |
|---|
| the DO NOT list | the DO list |
|---|
- DO NOT eat or drink in the laboratory.
- DO NOT drink or eat from science glassware.
- DO NOT place scientific equipment in your mouth.
- DO NOT store chemical substances in food containers such as bottles or jars.
- DO NOT return decanted substances to the original container as they may be contaminated.
- DO NOT put paper in the sink.
(put waste paper in the bin)
- DO NOT put broken glass in the sink or bin.
(use the sealed "sharps" container provided)
- DO NOT put heavy metals down the sink
(use the receptacle provided).
- DO NOT pour organic solvents down the sink
(use the sealed receptacle provided).
- DO NOT leave experiments unattended while in progress.
- DO NOT run in the laboratory.
|
- DO listen carefully and follow all intructions given by your teacher.
- DO wear appropriate Personal Protective Equipment (PPE)
- DO wash hands thoroughly after using chemicals.
- DO label all substances correctly in accordance with labelling guidelines.
- DO clean and keep tidy all surfaces where substances are used.
- DO clean glassware and other containers thoroughly after use.
- DO clean up mercury spills using the mercury spill kit or mercury decontaminant powder.
- DO clean up other spills using the appropriate materials available to you.
- DO dispose of all waste appropriately.
Excess acids and alkalis can be neutralised then flushed down the sink.
Non-hazardous solid waste, litmus paper, filter papers, matches, etc, go in the bin.
Broken glass goes in the "sharps" container.
Organic solvents go in the organic waste container.
Heavy metals go in the heavy metal container, except mercury waste which has a separate container.
(Your lab may also separate waste containing precious metals such as silver)
|
| DO NOT panic if there is an emergency! |
DO tell your teacher IMMEDIATELY if an emergency arises! |
Safety Equipment
Different laboratories will have different safety equipment depending on the type of experiments conducted in them.
You should not handle any safety equipment unless you have been given instructions about the correct use of the equipment.
Incorrect use of safety equipment can be dangerous!
Your teacher should be told immediately about any dangerous situation or emergency that arises during your work in the lab.
If you have not been given a sketch of the layout of the laboratory which includes the location of the safety equipment and emergency exits, you should draw a sketch yourself and include the locations of each of the following:
| Equipment in Fixed Positions |
Equipment in Non-fixed Positions |
|---|
- Emergency Exits
(look for a backlit EXIT sign above the door)
- Sand bucket
(usually near an exit door)
- Fire blanket
(usually near an exit door)
- Fire extinguishers
(usually near an exit door)
- First aid station
(usually attached to a wall near an exit door and a first aid poster)
- Eyewash facilities near first aid station
If eye washing facilities are not available, run low pressure water over the eyeball
(high pressure water can damage the eye)
- Fume hood (fume cupboard)
Used when handling substances that generate harmful gases, mists or dust
|
- Pipette bulbs (pi-pumps)
Only use dry pipette bulbs.
- Labels (and/or marking pens)
Correctly and legibly label all containers used to store substances
- Mercury absorption materials
Clean up mercury spills immediately and place residues in the sealed container provided.
- Spillage absorption materials
Clean up other hazardous liquid chemical spills using chemical absorbing agents and dispose of residues appropriately.
- Safety shield or screen
Used as a barrier to protect people from vigorous chemical reactions
- Trolleys
Used to transport heavy equipment including gas cylinders around the lab
- Winchester bottle carrier
Used to transport winchester bottles
|
Personal Protective Equipment (PPE)
Safety equipment designed to protect a single person (just you) from injury is known as personal protective equipment (abbreviated as PPE). Personal protective equipment includes wearing the appropriate clothing, shoes and accessories.
The personal protective equipment you should use in the chemistry laboratory is given below:
| Personal Protective Equipment |
Reason |
|---|
Laboratory Coats
or aprons |
Protects your clothes, and, provides an additional layer of protection for your skin.
(So always button-up your lab coat and DO NOT roll up the sleeves of your lab coat.) |
|
| Eye Protection |
Glasses or goggles with side shields to prevent substances entering your eyes. |
|
| Closed in shoes |
Protect the skin of your feet (no sandals, thongs or flip-flops). |
|
Hair ties
(hair nets) |
Long and/or loose hair must be secured to prevent it coming into contact with hazardous substances, and to prevent it getting jammed in machinery and endangering your life. Similarly, loose or large jewellery should be removed while working in the lab. |
|
| Gloves |
Protect your hands when necessary.
PVC or Nitrile gloves are used when handling toxic or corrosive substances.
Leather gloves are used to protect your skin from burns when using very hot, or very cold, substances. |
|
| Gauze masks |
Prevent hazardous dust entering your mouth and lungs and are used when necessary. |
Possible Hazards: Signage
Some hazards are general hazards, such as skidding on a wet floor, or tripping over your own feet when running. These hazards are present everywhere. It is important to be aware that these hazards are even more dangerous in the chemistry laboratory and you should take care to minimise the risk of injury due to these general hazards.
For example, electrical equipment, found in your home and in the laboratory, can be dangerous. To ensure you use electrical equipment safely, you should:
- keep electrical equipment away from water sources.
- prevent electrical cords being coiled as the build up of heat may cause a fire or damage the external (protective) casing.
- position electrical cords so that they do not pose a trip hazard.
- not use any equipment, power socket or power board which has a "Do Not Use" sign or label on it.
In the Chemistry lab there are other hazards, chemical hazards, that you may not find in other places, or, which become even more dangerous, even life-threatening in the context of the lab.
Hazardous chemical substances are labelled so that we can
- quickly identify the kind of risk or hazard associated with the substance
- take precautions to protect ourselves, and others, from the risk
The label on a container of a chemical reagent contains information about the risks or hazards associated with its use in the form of a
- pictogram (a picture representing the risk or hazard)(1)
- word (to describe the risk or hazard)
The pictogram and/or the word on the label of a hazardous substance summarises the risk involved in using the substance.
Suitable precautions should then be taken to minimize the risk when working with the substance.
The table below summarises the meaning of common pictograms and gives the typical safety precautions required to work with the substance.
Pictogram
Hazard |
Substance is |
Risk/hazard is |
Examples |
Typical Safety Precautions |
|---|
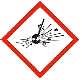
GHS01:Explosive
|
⚛ unstable explosives
⚛ self reactive substances
⚛ some organic peroxides
|
Substance will react quickly to produce huge amounts of gas in an explosion. |
Alkali metals reacting with water (lithium sodium, potassium, rubidium, caesium)
Ammonium dichromate
Gunpowder
2,4,6-Trinitrotoluene (TNT)
Nitroglycerin (dynamite, gelignite)
Picric acid
|
Use only small amounts of explosive materials.
Perform any experiment behind an explosion-proof barrier (closed fume hood or fume cupboard may be adequate in some cases).
Wear eye protection (glasses or goggles).
Wear suitable clothing, eg lab coat, closed in shoes, and gloves, to protect your skin. |
Pictogram
Hazard |
Substance is |
Risk/hazard is |
Examples |
Typical Safety Precautions |
|---|
|

GHS02:Flammable
|
⚛ flammable solid
⚛ flammable liquid
⚛ flammable gas
⚛ some organic peroxides
⚛ self-heating substances and mixtures
|
Substance will burn in the presence of oxygen and a heat source. |
Methane gas
Liquid alcohols like ethanol
Acetone (propanone)
Ethyl acetate (ethyl ethanoate)
Solid phosphorus
|
Keep the substance away from naked flames, for example, use a water bath, an oil bath, a heating mantle or hot plate to heat the substance safely. |
Pictogram
Hazard |
Substance is |
Risk/hazard is |
Examples |
Typical Safety Precautions |
|---|
|

GHS03:Oxidising
|
⚛ oxidising solid
⚛ oxidising liquid
⚛ oxidising gas
|
Can cause fires if they make contact with any combustible material. |
Solid potassium dichromate
Solid potassium permanganate
Concentrated nitric acid
Concentrated hydrogen peroxide
|
Keep away from combustible materials.
Perform oxidising experiments in fume cupboard.
|
Pictogram
Hazard |
Substance is |
Risk/hazard is |
Examples |
Typical Safety Precautions |
|---|
|

GHS04:Compressed Gas
|
⚛ compressed gas
⚛ liquefied gas
⚛ dissolved gas
|
Pressurized containers may burst if heated.
Refrigerated gases may cause cryogenic burns or injury.
|
Liquid nitrogen
Liquefied petroleum gas (LPG)
|
General: do not heat cylinder!
For liquefied gases like liquid nitrogen:
Wear insulating gloves to prevent contact with skin on hands.
Wear long sleeved lab coat to protect arms and torso.
Wear long pants to prevent contact with skin on legs.
Wear enclosed shoes to prevent contact with skin on feet.
Wear eye protection (safety glasses or goggles) to prevent contact with eyes.
|
Pictogram
Hazard |
Substance is |
Risk/hazard is |
Examples |
Typical Safety Precautions |
|---|
|
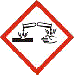
GHS05:Corrosive
|
⚛ corrosive to metals
⚛ corrosive to skin
⚛ damaging to eyes
|
Substance will eat away at your skin and flesh. |
Acids
(eg hydrochloric acid)Alkalis
(eg sodium hydroxide) Some gases
(eg chlorine, ammonia) Batteries containing acid. |
Wear gloves to prevent skin contact with the substance through your hands.
Wear a long-sleeved lab coat to protect your arms and torso.
Wear eye protection (glasses or goggles) to prevent substance entering your eyes.
Wear closed in shoes to protect the skin of your feet. |
Pictogram
Hazard |
Substance is |
Risk/hazard is |
Examples |
Typical Safety Precautions |
|---|
|

GHS06:Toxic
|
⚛ acutely toxic (oral, dermal, inhalation) |
Substance will cause sickness or death if it enters your body. |
Cyanides Lead acetate Arsenic compounds Pesticides |
Wear gloves, lab coat and closed in shoes to prevent the substance being absorbed through your skin.
Use a fume hood (fume cupboard) to prevent breathing any toxic fumes (gases). |
Pictogram
Hazard |
Substance is |
Risk/hazard is |
Examples |
Typical Safety Precautions |
|---|
|

GHS07:Harmful
|
⚛ skin irritant
⚛ eye irritant
⚛ skin sensitization
⚛ respiratory tract irritant
⚛ narcotic effect
|
Substance is an irritant, can cause skin to itch, eyes to water, coughing, etc |
Solid copper sulfate (skin irritant)
Styrene (phenylethene) (produces an irritating vapour)
|
Protect skin using gloves, lab coats and covered shoes.
If the irritant is a vapour, perform experiments in fume cupboard.
|
Pictogram
Hazard |
Substance is |
Risk/hazard is |
Examples |
Typical Safety Precautions |
|---|
|

GHS08:Health Hazard
|
damaging to genetic material :
⚛ mutagens
⚛ carcinogens
⚛ reproductive toxicity
⚛ respiratory sensitisers
⚛ aspiriaction hazard
|
(i) Substance contains a virus or bacteria that can cause you serious health problems, even death. |
Vaccines Hepatitis |
Wear suitable clothing such as lab coat, closed in shoes, eye protection (glasses or goggles), gloves, to prevent contact through your skin.
Use a fume hood or fume cupboard to prevent breathing in the hazardous material. |
| (ii) Substance gives off radiation which can destroy your cells, cause burns, and could lead to cancer. |
Uranium oxide Radiopharmaceuticals used to treat cancer |
Any radioactive substance you will use in School will come in special packaging.
Do not remove a radioactive substance from its packaging.
Radioactive sources should be handled with tongs or forceps and kept at least 30 cm from any person.
Minimize the length of time you are exposed to the radiation by keeping the radioactive source in a lead lined container when you are not using it. |
Pictogram
Hazard |
Substance is |
Risk/hazard is |
Examples |
Typical Safety Precautions |
|---|
|
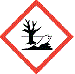
GHS09:Environmental Hazard
|
⚛ an actue or chronic hazard to aquatic environments
|
Damages things living in water in the short-term and/or in the long-term. |
Heavy metals such as mercury and lead. |
Put environment-damaging waste into a suitable storage vessel (often a labelled winchester bottle) for appropriate disposal at a later date.
|
Possible Hazards: Glassware
You will be handling a lot of glassware during your chemistry course.
Minor accidents, such as cuts, can be avoided if you are observant.
If any glass is broken in the laboratory, DO NOT pick up the pieces with your bare hands, use a broom to sweep the glass fragments up into a pan, then empty the pan into the glass bin.
DO NOT place glass in the bin used for general waste.
Carefully examine glassware for chips or cracks before you use it.
NEVER use cracked or chipped glassware. Dispose of the glassware in the glass bin.
"Star" cracks can be quite small but can rapidly and suddenly expand if the glassware is heated or placed under vacuum. Dispose of the glassware in the glass bin.
Pasteur pipettes or micropipettes are easily broken and can be sharp.
DO NOT leave these on a bench, always store them in a small beaker when you are not using them.
When using fitted glassware, such as ground-glass male-and-female connections, do not use force to insert the "male" into the "female".
To correctly insert a thermometer into an adapter such as is used for distillation:
- Turn the screw cap to make a wide aperture so that the thermometer can slide in easily.
- Hold the thermometer close to the "bulb" or "reservoir" NOT at the very end of the thermometer and insert it through the screw cap.
- When the thermometer is in place, screw the screw cap firmly but without over tightening.
To correctly attach rubber tubes to water-cooled (Liebig) condensers:
- Grip the condenser so that the "arm" protrudes through your fingers.
- Gently insert the "arm" into the rubber tubing. DO NOT use force.
If your condenser has plastic connectors:
- Undo the connectors first.
- Attach the rubber tubes.
- Screw the connectors back into place.
To correctly attach a pipette filler to a pipette always hold the pipette close to where the filler will attach and DO NOT force to insert the pipette into the filler.
Worked Example of How to Work Safely in the Lab: Heating a solid
In a typical thermal decomposition experiment, a small amount of calcium carbonate is placed in a test tube and heated in a bunsen burner flame to produce carbon dioxide gas and calcium oxide.
What safety precautions should be taken during this experiment?
Question 1: Do you know the safety rules for working in this laboratory?
| Read through the Safety Rules before you go to the lab to familiarise yourself with the rules. |
Question 2: Do you know where the safety equipment is located in this laboratory?
| Take a look at the sketch map of the lab and make sure you know where safety equipment is located.
Make sure that you are wearing appropriate clothing before you go to the lab. |
Question 3: What are the possible hazards?
| Hazard |
|
|
|
|
|---|
| Heat source |
|
|
|
|
|
| Hot materials |
|
|
|
|
|
| Explosion |
(if any gas is produced quickly in a confined space an explosion is possible) |
|
| Corrosive product |
(calcium oxide produces an alkali when it comes into contact with water) |
Question 4: What are the possible consequences of these hazards (the risks)?
| Hazard |
Consequence
(risks) |
|
|
|
|---|
| Heat source |
burns |
|
|
|
|
| Hot materials |
burns |
|
|
|
|
| Explosion |
skin, eye and hearing damage |
|
|
|
|
| Corrosive product |
skin and eye damage |
|
|
|
Question 5(i): Is it possible to eliminate the risk?
| Hazard |
Consequence
(risks) |
Eliminate Risk? |
|
|
|---|
| Heat source |
burns |
No. Heat source is required. |
|
|
|
| Hot materials |
burns |
No. Materials must be heated. |
|
|
|
| Explosion |
skin, eye and hearing damage |
Yes.
(i) Heat gently (slowly move test tube in and out of flame).
(ii) Use an open-mouthed test tube (do not restrict the opening) |
|
| Corrosive product |
skin and eye damage |
No. Calcium oxide will be produced in air which contains water. |
|
|
Question 5(ii): Is it possible to substitute for a safer alternative?
| Hazard |
Consequence
(risks) |
Eliminate Risk? |
Substitute? |
|
|---|
| Heat source |
burns |
No |
No. Heat source is required. |
|
|
| Hot materials |
burns |
No |
No. Materials must be heated. |
|
|
| Explosion |
skin, eye and hearing damage |
Yes.
(i) Heat gently (slowly move test tube in and out of flame).
(ii) Use an open-mouthed test tube (do not restrict the opening) |
|
| Corrosive product |
skin and eye damage |
No |
No. Experiment requires calcium oxide be produced. |
|
Question 5(iii): Is it possible to guard against, that is, to minimise, the risk?
| Hazard |
Consequence
(risks) |
Eliminate Risk? |
Substitute? |
Minimise Risk? |
|---|
| Heat source |
burns |
No |
No |
Yes.
(i) Convert blue bunsen burner flame to a luminous (yellow) flame when not in use, or, turn it off.
(ii) Do not set bunsen burner up near edge of bench or close to another experiment.
(iii) Do not place equipment, including pens or lab book, near the bunsen burner.
(iv) Do not leave lit bunsen burner unattended.
|
|
| Hot materials |
burns |
No |
No |
Yes
(i) Wear a lab coat.
(ii) Wear eye protection.
(iii) Wear closed in shoes.
(iv) Use tongs or test tube holder to hold test tube while heating.
(v) Slant open mouth of the test tube away from your body or other people while heating.
(vi) Place hot test tube in test tube rack to cool it before disposing of waste.
(vii) Allow bunsen burner to cool before storing it away.
|
|
| Explosion |
skin, eye and hearing damage |
Yes.
(i) Heat gently (slowly move test tube in and out of flame).
(ii) Use an open-mouthed test tube (do not restrict the opening)
|
|
| Corrosive product |
skin and eye damage |
No |
No |
Yes
(i) Do not touch the substances.
(ii) Add some dilute acid to the calcium oxide to neutralise it before pouring it down the sink.
(iii) Wash your hands after you finish cleaning up the experiment.
(iv) Wear a lab coat.
(v) Wear eye protection.
(vi) Tie back loose/long hair
(vii) Remove loose/long jewellery or other accessories.
|