13C Nuclear Magnetic Resonance Spectroscopy Tutorial
Key Concepts
- Nuclear Magnetic Resonance Spectroscopy, or NMR Spectroscopy, can be used to identify any isotope, unless the isotope has both an even number of protons and an even number of neutrons.
- The nuclei of many elements, such as 13C, spin generating a magnetic field.
When placed in a strong external field they align themselves either with or against the field.
When the sample is irradiated with radio waves the energy absorbed corresponds to the difference between these two magnetic alignments. - 13C NMR Spectroscopy (carbon nuclear magnetic resonance spectroscopy) is used to identify the structure of organic (carbon) compounds.
13C NMR spectra provide information about:
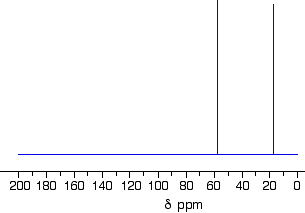
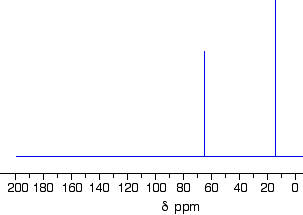
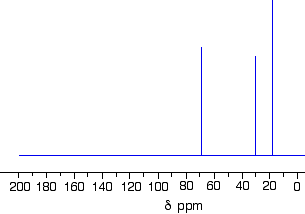
- The Number of Signals: each chemically different carbon in a structure is also magnetically different.
CH3 groups are chemically different to CH2 groups and to CH groups.
eg, CH3CH2CH2Cl contains 3 chemically different environments for the carbon atoms:
CH3
CH2 adjacent to CH3
CH2 covalently bonded to Cl - The Position of the Signal with respect to an internal standard (chemical shift): tetramethylsilane, (CH3)4S or TMS, is often used as an internal standard since almost all 13C signals appear downfield from the TMS signal.
On the δ scale, the TMS signal is 0 ppm
Tables of chemical shifts are derived from measurements of a large number of samples and represent a "normal" range.
13C signals affected by highly electronegative elements are shifted further downfield.