Electroplating Concepts Chemistry Tutorial
Key Concepts
- Electroplating is also known as electrodeposition.
- Electroplating uses an electrolytic cell to deposit a coating of one metal onto another metal.
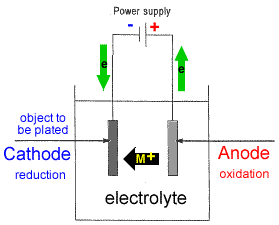
- Setting up a typical electrolytic cell for electroplating:
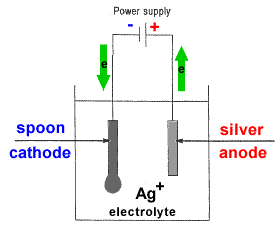
- The object to be plated (coated with a different metal) is made the cathode (negative electrode) by connecting it to the negative terminal of the power supply.
- The metal to be plated onto the object is made the anode (positive electrode) by connecting it to the positive terminal of the power supply1.
- The electrolyte contains the metallic ions of the metal to be plated onto the object.
The electolyte may be either a liquid (molten salt) or an aqueous solution, but is usually an aqueous solution.
- Electrochemical Processes:
- At the anode (positive electrode), metal ions are oxidized and enter the electrolyte as mobile ions:
M(s) → M+ + e-
The anode will disintegrate in time as the metal atoms, M(s), are converted to metal ions, M+. - At the cathode (negative electrode), metal ions from the electrolyte are reduced:
M+ + e- → M(s)
Solid metal, M(s), is deposited on the cathode. - The concentration of metal ions, [M+], in the electrolyte should remain constant during this process since just as many cations are being produced at the anode as are being used up at the cathode2.