Naming Inorganic Non-Metallic Binary Covalent Compounds Chemistry Tutorial
Key Concepts
- A binary compound is made up of two different elements.
This tutorial looks at binary compounds in which both elements are non-metals.
- A binary covalent compound refers to two different elements which are joined together by covalent bonds in a molecule.
- Inorganic compounds are, in general, compounds that do not contain carbon.(1)
There are notable exceptions such carbon monoxide (CO) and carbon dioxide (CO2) for example.
- Inorganic non-metallic binary covalent compounds can be named based on the composition of the compound (referred to as compositional nomenclature(2)).
- The name of an inorganic non-metallic binary covalent compound using compositional nomenclature tells us:
⚛ which two elements are present in a molecule of the compound
⚛ number of atoms of each element present in a molecule of the compound
- Using compositional nomenclature, non-metallic elements in inorganic binary covalent compounds are named in the following order from the element named first to the element named last:(3)
carbon, phosphorus, nitrogen, hydrogen, sulfur, oxygen, iodine, bromine, chlorine, fluorine
Apart from hydrogen, this order is based on the non-metallic element's location in the periodic table as shown below: (4)
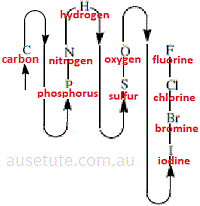
(Start with carbon, C, then follow the arrows around to fluorine, F )
- In the name of the inorganic non-metallic binary covalent compound, the element that is:
(a) named first retains its name (the name is not modified)
(b) named last has a modified name ending in "ide" as shown in the table below:
element's
namemodified name
of elementflourine fluoride chlorine chloride bromine bromide iodine iodide oxygen oxide sulfur sulfide hydrogen hydride nitrogen nitride phosphorus phosphide carbon carbide - A multiplicative prefix(5) precedes the name of the element, or modified name of the element, and is joined directly to the name (no spaces nor hyphens between the prefix and the name) in order to tell us how many atoms of this element are present in a molecule of the inorganic non-metallic covalent compound:
No. of atoms 1 2 3 4 5 6 7 8 9 10 Multiplicative prefix mono di tri tetra penta hexa hepta octa nona deca - Using compositional nomenclature the two parts of the name of the inorganic non-metallic binary covalent compound are separated by a space:
first part last part prefixname prefixnamide - Exceptions to the IUPAC recommendations for naming inorganic non-metallic binary covalent compounds:
(i) The final vowel(6) of the multiplicative prefix should NOT be elided (should NOT be removed).
The exception is that if 1 oxygen atom is present it is usually named as a monoxide NOT as a monooxide.
IUPAC allows this exception because the form "monoxide" has been in general useage for a very long time.
Note that both versions of the name, monoxide AND monooxide are acceptable IUPAC names.(ii) The prefix mono is not used in the final name of the compound UNLESS the name could be ambiguous without it (could apply to more than one compound).(7) Even when more than one compound can be formed using the same two elements in different ratios, then mono prefix is often dropped.
For example carbon monoxide is the name of the compound with the fomula CO and carbon dioxide is the name of the compound with the formula CO2.
1 atom of carbon is present in both molecules so we don't need to write monocarbon.
The number of oxygen atoms does differ in the two compounds so we distinguish between the two using the di and tri multiplicative prefixes before the name oxide.
If in doubt it is better to retain the "mono" prefix, it won't be wrong, but it may not be the name that is in common useage by chemists.(iii) Some compounds have names that are accepted by IUPAC even though they are not systematically named. Examples you might encounter during your chemistry course include:(8)
⚛ water for H2O (not dihydrogen monoxide)
⚛ hydrogen peroxide or dihydrogen peroxide for H2O2 (not dihydrogen dioxide)
⚛ either hydrogen sulfide or dihydrogen sulfide is an acceptable IUPAC name for H2S
⚛ ammonia for NH3 (not nitrogen trihydride)
(iv) Compositional names are not the preferred naming system for some compounds. Examples you might encounter in your chemistry course include:
⚛ diazene for N2H2 (not dinitrogen dihydride)
⚛ hydrazine for N2H4 (not dinitrogen tetrahydride)
