Addition Polymerisation Reaction
Polystyrene is produced in an addition polymerisation reaction from styrene monomers.
| styrene | → | polystyrene |
| H
| | | 
| |
| n | C | = | C |
| |
H | | |
H |
|
→ |
| | H
| | | 
| | | H
| | | 
| | | H
| | | 
| | | H
| | | 
| | | H
| | | 
| | | H
| | | 
| | | H
| | | 
| | | |
| -[ | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | ]n- |
| | |
H | | |
H | | |
H | | |
H | | |
H | | |
H | | |
H | | |
H | | |
H | | |
H | | |
H | | |
H | | |
H | | |
H | | |
|
The reaction is highly exothermic, the heat of reaction for the polymerisation is -121 kJ mol-1 (at 25oC).
styrene → polystrene ΔH = - 121 kJ mol-1
By Le Chatelier's Principle, increasing the temperature at which the reaction occurs would favour the reactant, monomer, side of the equation.
The addition polymerisation reaction is therefore carried out at very mild temperatures.
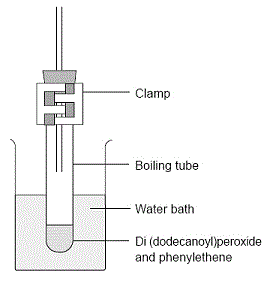
Polystyrene can be produced in the school laboratory as a demonstration.
| Set up a water bath using a 250 mL of boiling water.
Add 0.1 g of di(dodecanoyl) peroxide to 5 mL of styrene4 in a boiling tube.
Insert 20 cm of glass tubing through a rubber bung (stopper) and position the rubber bung in the neck of the flask as shown in the diagram. This minimises the loss of escaping styrene vapour during heating.
Position the boiling tube so that the level of the solution in it is below the level of the hot water and clamp it in place.
Heat for 30 minutes until the solution becomes viscous.
Extinguish all flames and remove the boiling tube from the water bath and cool.
Pour the contents of the cooled boiling tube into 50 mL of ethanol in a beaker.
Push the polystyrene into a lump using a glass rod.
Pour off the ethanol.
Dry the solid polystyrene on filter paper.
|
| Safety Considerations |
|---|
| Wear eye protection (safety glasses or goggles) and disposable gloves.
Styrene vapour is narcotic at high concentrations, Work in a fume cupboard, or, ensure good ventilation.
Styrene is flammable, keep clear of flames.
Di(dodecanoyl) peroxide (lauroyl peroxide) is an oxidising agent, avoid contact with skin, benches etc.
Ethanol is flammable, keep clear of flames.
Polystyrene is flammable, keep clear of flames.
|
|
You could test the solubility of your polystyrene in various solvents such as concentrated hydrochloric acid (12 mol L-1), dilute hydrochloric acid (10% HCl(aq)), cyclohexane, olive oil, dichloromethane, bromine and 2-butanone.
Reaction Mechanism
The addition polymerisation of styrene (ethenylbenzene or phenylethene) to produce polystyrene (poly(phenylethene) or poly(ethenylbenzene)) proceeds by a free-radical mechanism.
A free radical is a molecule that has no charge, but, is highly reactive because it has an unpaired valence electron.
Di(dodecanoyl) peroxide can be used as an initiator for the polymerisation reaction because it has a peroxy group (-O-O-) between two large dodecanoyl groups (CH3(CH2)10CO-).
The peroxy O-O bond breaks readily, splitting the di(dodecanoyl)peroxide molecule into two, and leaving unpaired electrons on the oxygen atoms.
This results in the formation of the free radical CH3(CH2)10COO.
| di(dodecanoyl) peroxide | → | free radical |
| | O
|| | | O
|| | |
| CH3(CH2)10 | C | -O-O- | C | (CH2)10CH3 |
|
heat
→ |
|
When writing chemical equations for polymerisation reactions involving a free radical initiator, Chemists usually use the symbol R. for the free radical.
These free radicals attack the styrene (phenylethene or ethenylbenzene) molecules so that the double bond opens up resulting in unpaired electrons at the end of the growing polymer chains.
Initiation: free radical initiator attacks the styrene monomer, opening the double bond and producing a new free radical with an unpaired electron on a carbon atom
| R. |
+ |
H
| | | 
| |
| C | = | C |
|
H | | |
H |
|
→ |
| H
| | | 
| |
| R- | C | - | C. |
| |
H | | |
H |
|
Propagation: the new free radical produced during initiation can then react with a styrene molecule, opening up the double bond and leaving an unpaired electron on a carbon atom
| H
| | | 
| |
| R- | C | - | C. |
| |
H | | |
H |
|
+ |
H
| | | 
| |
| C | = | C |
|
H | | |
H |
|
→ |
| H
| | | 
| | | H
| | | 
| | |
| R- | C | - | C | - | C | - | C. | etc |
| |
H | | |
H | | |
H | | |
H | |
|
This newly produced free radical is then able to react with another styrene molecule, and so on and so forth, building up a long polymer chain.
Termination: polymerisation stops when two free radicals react with each other
| R. |
+ |
| H
| | | 
| | | H
| | | 
| | |
| R- | C | - | C | - | C | - | C. | |
| |
H | | |
H | | |
H | | |
H | |
|
→ |
| H
| | | 
| | | H
| | | 
| | |
| R- | C | - | C | - | C | - | C | -R |
| |
H | | |
H | | |
H | | |
H | |
|
This is why polymer chains are of varying lengths, any two of the free radicals produced at any stage during the reaction can react and terminate the reaction. So a short chain might react with a longer chain, or two short chains might react, or two long chains, or a chain might react with the free radical used to initiate the reaction (R.) as shown above.
Structure of Polystyrene
There are a number of different ways in which styrene (ethenylbenzene or phenylethene) molecules could combine together to form long polymer chains.
1. All the styrene molecules combine so that the benzene rings ( ) are all on the same side of the carbon backbone of the polymer chains:
) are all on the same side of the carbon backbone of the polymer chains:
| H
| | | 
| | | H
| | | 
| | | H
| | | 
| | | H
| | | 
| | | H
| | | 
| | | H
| | | 
| | | H
| | | 
| | |
| - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - |
| |
H | | |
H | | |
H | | |
H | | |
H | | |
H | | |
H | | |
H | | |
H | | |
H | | |
H | | |
H | | |
H | | |
H | |
This structure is known as isotactic polystyrene.
The regular arrangement of the benzene rings in this structure allows the polymer chains to pack together closely and maximises the intermolecular forces between the chains.
Close packing reduces the flexibility of the material, so isotactic polystyrene would be quite rigid, and because the intermolecular forces between polymer chains is maximised, it would also be quite strong.
Isotactic polystyrene is said to be highly crystalline.
Although we commonly like to draw the structure of polystyrene as the isotactic structure because it is easy to see the repeating units, in fact, when styrene monomers are polymerised very little of the resulting polystyrene is in the isotactic form.
2. The styrene (ethenylbenzene or phenylethene) molecules combine so that the benzene rings ( ) alternate between being above the plane of the carbon backbone and being below it:
) alternate between being above the plane of the carbon backbone and being below it:
| H
| | | H
| | | H
| | | 
| | | H
| | | H
| | | H
| | | 
| | | H
| | | H
| | | H
| | | 
| | | H
| | | H
| | |
| - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - |
| |
H | | |
 | | |
H | | |
H | | |
H | | |
 | | |
H | | |
H | | |
H | | |
 | | |
H | | |
H | | |
H | | |
 | |
This structure is known as syndiotactic polystyrene.
The regular arrangement of the benzene rings in this structure allows the polymer chains to pack together closely and be held in place by intermolecular forces between the chains.
Close packing reduces the flexibility of the material, so syndiotactic polystyrene is quite rigid, and the action of intermolecular forces between polymer chains makes it quite strong.
Syndiotactic polystyrene, like isotactic polystyrene, is said to be highly crystalline.
Very little of the polystyrene produced by the addition polymerisation of styrene (ethenylbenzene or phenylethene) is syndiotactic polystyrene.
However, Chemists think syndiotactic polystyrene could be used to make medical equipment because it is able to withstand the heat, moisture and cleaners used to sterilize medical equipment, but it is currently quite expensive to produce.
3. The styrene (ethenylbenzene or phenylethene) molecules combine so that benzene rings ( ) are randomly oriented along the chains, with some above and some below the plane of the carbon backbone:
) are randomly oriented along the chains, with some above and some below the plane of the carbon backbone:
| H
| | | H
| | | H
| | | 
| | | H
| | | 
| | | H
| | | 
| | | H
| | | H
| | | H
| | | 
| | | H
| | | H
| | |
| - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - | C | - |
| |
H | | |
 | | |
H | | |
H | | |
H | | |
H | | |
H | | |
H | | |
H | | |
 | | |
H | | |
H | | |
H | | |
 | |
This structure is known as atactic polystyrene.
The large benzene rings sticking out at random along the chains prevent the polymer chains from packing together closely.
Atactic polystyrene is not crystalline, rather, it is said to be amorphous. Non-crystalline or amorphous polymers are expected to be softer and more flexible.
Most of the polystyrene produced by the addition polymerisation of styrene is atactic polystyrene.
Atactic polystyrene is the polystyrene you will find in polystyrene containers, cases, cups, plastic cultery etc.
Properties and Uses of Polystyrene
Polystyrene is a linear polymer, and, like most linear polymers, the application of heat and pressure will cause it soften and take on new shapes. These linear polymers are said to be thermoplastic. Polystyrene is a thermoplastic.
| Property |
Poly(phenylethene)
(polystyrene) |
Uses |
|---|
| Melting Point |
240oC (softens at ~100oC) |
Thermoplastic with low softening point enables it to be easily molded.
|
|
| Crystallinity |
Irregular packing and low crystallinity (amorphous) of atactic polymer chains |
|
|
| Flexibility |
rigid (or foamed) |
Polystyrene items hold their shape but are usually quite brittle.
Adding rubbers such as polybutadiene makes the polymer more flexible and these materials are usually referred to as high-impact polystyrene.
|
|
| Heat Resistance |
good (polystyrene foam has better heat resistance) |
Foam used to make heat insulation.
Take-away coffee cups are often made of polystyrene foam. |
|
| Transparency |
transparent |
|
|
| Density |
~0.96 - 1.04 g cm-3 |
Low density foam used to make flotation devices. |
|
| Chemical Properties |
Resistant to acid, alkali and water.
Dissolves in many chlorinated solvents |
Polystyrene is suitable for use in food containers, cutlery.
|
1 The IUPAC name for linear-chain polymers is made by placing the prefix poly before the name of the constitutional repeating unit in parentheses. However, the name of the repeating unit could be based on its source, poly(ethenylbenzene), or, on the structure of the polymer, poly(1-phenylethane-1,2-diyl). Luckily, some well-established common names, like polystyrene, are still acceptable. Refer to the IUPAC webiste for a guide to polymer nomenclature (A Brief Guide to Polymer Nomenclature (2012 pdf).
2Dow Chemical invented the Styrofoam process in 1941.
3Polystyrene is inexpensive to produce, so the cost of recycling it needs to be very low in order to make recycling commercially viable. This is particularly true for expanded polystyrene (EPS) or polystyrene foam because the 'gas bubbles' cause problems in the recycling process.
4Styrene, or ethenylbenzene or phenylethene, may contain the inhibitor 4-(dimethylethyl)-benzene-1,2-diol (4-tert-butyl catechol) which needs to be removed by washing with 1 mol L-1 NaOH(aq) then with water in a separating funnel. Dry the phenylethene over anhydrous sodium sulfate, Na2SO4(s), for 10 minutes. Wash all equipment in propanone (acetone), CH3COCH3.

