Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Theory
Preparing the Wine Sample for Analysis
The ethanol content of wine can be as high 16%(v/v) which is quite a concentrated solution. So the wine is first prepared by diluting it.
1:25 dilution used in Experiment 1 (oxidation to ethanal)
- Use a pipette to place a 10.0 mL aliquot of wine into a 250.0 mL volumetric flask and make up to the mark with water.
- Use a pipette to transfer 20.0 mL of this diluted wine to a conical flask.
This conical flask will be used in the next step in which the ethanol is oxidised.
- It is important to remember that you have diluted the original wine.
When you come to calculate the concentration of the ethanol in the wine later on you will need to remember that the ethanol concentration in the wine will be much greater than the concentration of ethanol in the sample you use in your titration.
(250/10 = 25 times greater!)
1:50 dilution used in Experiment 2 (oxidation to acetic acid)
- Use a pipette to place a 20.0 mL aliquot of wine into a 1.0 L volumetric flask and make up to the mark with water.
- Use a pipette to transfer 1.0 mL of this diluted wine to a sample holder.
This ethanol is to be oxidised in the next step.
- It is important to remember that you have diluted the original wine.
When you come to calculate the concentration of the ethanol in the wine later on you will need to remember that the ethanol concentration in the wine will be much greater than the concentration of ethanol in the sample you use in your titration.
(1000/20 = 50 times greater!)
Oxidising the Ethanol
- Potassium dichromate (K2Cr2O7) is an oxidising agent, it causes something else (the ethanol) to be oxidised.
In this reaction chromium will be reduced from an oxidation state of +6 in Cr2O72- to +3 in Cr3+.
- The potassium dichromate solution must be acidified by adding concentrated sulfuric acid.
A source of protons, H+, is required for this redox reaction. Concentrated sulfuric acid is providing the protons.
- Ethanol is highly volatile, on heating the ethanol will evaporate.
We need to ensure that ALL of the ethanol (C2H5OH) in the wine sample is oxidised to ethanal (CH3CHO) in experiment 1, or to acetic acid (CH3COOH) in experiment 2.
In experiment 1, the flask is stoppered to prevent the ethanol being lost and the overall redox reaction is:
| 3C2H5OH(aq) |
+ |
Cr2O72-(aq) |
+ |
8H+ |
→ |
3CH3CHO(aq) |
+ |
2Cr3+(aq) |
+ |
7H2O |
In experiment 2, a small wine sample is warmed overnight to evaporate all the ethanol and trap it in a sealed flask with the dichromate, and, the next morning the sides washed down with water to ensure all the ethanol in washed into the flask.
The overall redox reaction equation is:
| 3C2H5OH(aq) |
+ |
2Cr2O72-(aq) |
+ |
16H+ |
→ |
3CH3COOH(aq) |
+ |
4Cr3+(aq) |
+ 11H2O |
Note that you solution should NOT be green!
Because we need to add an excess of the orange dichromate solution, the solution in our flask after all the ethanol has been oxidised should still be orange (or possibly a darker red-orange colour).
The concentration of ethanol in the conical flask in either experiment 1 or 2 is now 0
The conical flask contains excess dichromate ions (Cr2O72-), excess H+, K+ spectator ions, as well as the products of the reaction chromium(III) ions (Cr3+) and either ethanal (experiment 1) or acetic acid (experiment 2).
Reacting Excess Dichromate with Iodide
Potassium iodide is added to the flask (both experiments 1 and 2).
The excess dichromate ions will oxidise iodide ions, I-, to iodine atoms in I2:
oxidation: 2I- → I2 + 2e-
while the iodide ions reduce the dichromate ions to chromium(III) ions:
reduction: Cr2O72- + 14H+ + 6e- → 2Cr3+ + 7H2O
The overall redox reaction equation:
| reduction: |
Cr2O72- |
+ |
14H+ |
+ |
6e- |
→ |
2Cr3+ |
+ |
7H2O |
|
|
| oxidation: |
|
|
|
|
6I- |
→ |
6e- |
|
|
+ |
3I2 |
|
|
redox
equation: |
Cr2O72- |
+ |
14H+ |
+ |
6I- |
→ |
2Cr3+ |
+ |
7H2O |
+ |
3I2 |
By adding an excess of potassium iodide we ensure that all the excess dichromate ions will react with the iodide ions to form iodine.
The solution will look rather brown due to the presence of the I2.
The colour of this solution can be used to indicate the end-point of the titration with sodium thiosulfate, which is the the next step.
Titration with Thiosulfate
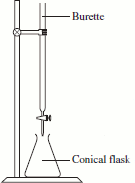
- Fill a burette with a standard sodium thiosulfate solution (Na2S2O3).
- Titrate the contents of the conical flask (from above) with the thiosulfate solution.
Balanced redox equation:
I2(aq) + 2S2O32-(aq) → 2I-(aq) + S4O62-(aq)
Note that the presence of I2 in the solution will make it appear brown.
Thiosulfate ions act as a reducing agent, reducing I2 to colourless I- and thereby removing the brown colour from the solution.
- Record the titre.
- Repeat the experiment several times until concordant titres are produced.
The average titre becomes the basis for calculating the concentration of ethanol in the alcholic beverage.
Experiment 1: Oxidation to Alkanal Method
This method gives good results for the experimental determination of ethanol content in white wine.
The colour of red wine or beer will mask the end point of the titration.
The concentration of ethanol (ethyl alcohol, C2H5OH) is to be determined using a redox back titration method.
Procedure:
- Pipette 10.0 mL of wine into a 250.0 mL volumetric flask.
- Add distilled water to make up to 250.0 mL and mix thoroughly.
- Pipette 20.0 mL of diluted wine into a conical flask.
- Add 20.0 mL aliquot of 0.04 mol L-1 potassium dichromate solution.
- Add 10 mL concentrated sulfuric acid (40%)
- Place stopper in flask loosely and heat in a 45oC water bath for 10 minutes then remove from heat.
- Add 2 g potassium iodide.
- Titrate solution with 0.010 mol L-1 sodium thiosulfate solution under solution clears.
- Add 1 mL starch solution and continue titrating until blue colour disappears and solution is clear green.
- Record the volume of sodium thiosulfate (the titre).
- Repeat procedure on several samples of the diluted wine until concordant titres are achieved.
|
| Safety Considerations |
|---|
- Sulfuric acid is corrosive.
Wear safety glasses (goggles)
- Avoid contact with iodine.
Iodine stains skin, clothing & benches.
- Potassium dichromate is a carcinogen.
Avoid contact with skin.
|
|
Calculations:
- average titre (mL of Na2S2O3)
- moles of S2O32- used in titration with dichromate ions.
- moles of I2 present in the flask at the time of the titration.
- moles of Cr2O72- in the flask at the time of titration.
- moles of Cr2O72- added to the diluted wine sample at the start of the experiment.
- moles of Cr2O72- that reacted with ethanol.
- moles of C2H5OH in the diluted wine sample that reacted with Cr2O72-
- concentration of C2H5OH in diluted wine sample.
- concentration of C2H5OH in original, undiluted wine.
Calculating the Ethanol Content in Wine in mol L-1
10 mL of wine was placed in a 250 mL volumetric flask and water was added up to the mark.
20.0 mL of the diluted wine was placed in a conical flask with 20.0 mL of 0.04 mol L-1 K2Cr2O7(aq) and sulfuric acid to acidify the solution.
After gentle heating, 2 g of KI(s) was added and the solution titrated with 0.010 mol L-1 Na2S2O3(aq).
The results of the experiment are shown below:
| sample | volume of sodium thiosulfate solute
(titre) |
|---|
| A | 31.92 mL |
| B | 29.63 mL |
| C | 29.61 mL |
Calculation: concentration of ethanol in the wine.
- Calculate the average titre:
Disregard the first titre as being a "rough" titration to help establish the approximate end point.
average titre = (29.63 + 29.61) ÷ 2 = 29.62 mL of sodium thiosulfate solution.
- Calculate the moles of sodium thiosulfate that reacted with the iodine:
concentration (mol L-1) = moles ÷ volume (L)
moles (Na2S2O3) = concentration (mol L-1) × volume (L)
concentration (Na2S2O3) = 0.01 mol L-1 (given in the steps above)
volume (Na2S2O3) = average titre = 29.62 mL = 29.62 mL ÷ 1000 mL/L = 0.02962 L
moles (Na2S2O3) = 0.01 × 0.02962 = 2.96 × 10-4 mol
- Calculate the moles of iodine (I2) present in the flask that have reacted with the thiosulfate solution (S2O32-):
Balanced redox equation:
I2(aq) + 2S2O32-(aq) → 2I-(aq) + S4O62-(aq)
1 mole of I2 reacts with 2 moles of S2O32-
therefore ½ mole I2 reacts with 1 mole S2O32-
so, 2.96 × 10-4 mol S2O32- reacted with ½ × 2.96 x 10-4 = 1.48 × 10-4 moles of I2
moles of I2 = 1.48 × 10-4 mol
- Calculate the moles of dichromate that were in excess in the flask:
Balanced redox equation:
Cr2O72-(aq) + 14H+ + 6I-(aq) → 2Cr3+(aq) + 7H2O(l) + 3I2(aq)
1 mole dichromate (Cr2O72-) produces 3 moles I2
therefore 1 mole I2 is produced by 1/3 mole Cr2O72-
so, 1.48 × 10-4 mol I2 is produced by 1/3 × 1.48 x 10-4 = 4.94 × 10-5 mol of Cr2O72-
4.94 × 10-5 mol of Cr2O72- was present in the flask AFTER all the ethanol had been oxidised.
- Calculate how much dichromate was added to the sample intially:
concentration (mol L-1) = moles ÷ volume (L)
moles = concentration (mol L-1) × volume (L)
concentration (Cr2O72-) = 0.04 mol L-1 (give in the steps above)
volume (Cr2O72-) = 20.0 mL (given in the steps above) = 20.0 mL ÷ 1000 mL/L = 0.020 L
moles (Cr2O72-) = 0.04 × 0.020 = 8.0 × 10-4 mol
- Calculate the moles of dichromate in excess in the flask (after all the ethanol has been oxidised)
moles (Cr2O72-) added = 8.0 × 10-4 mol
moles (Cr2O72-) in excess (that is, moles that then reacted with I-) = 4.94 × 10-5 mol
moles (Cr2O72-) that reacted with ethanol = moles added to flask - moles in excess = 8.0 × 10-4 - 4.94 × 10-5 = 7.51 × 10-4 mol
moles Cr2O72- that reacted with ethanol = 7.51 × 10-4 mol
- Calculate the moles of ethanol that reacted with dichromate in the flask:
Balanced redox equation:
3C2H5OH(aq) + Cr2O72-(aq) + 8H+ → 3CH3CHO(aq) + 2Cr3+(aq) + 7H2O
3 moles ethanol (C2H5OH) reacts with 1 mole dichromate (Cr2O72-)
so, 7.51 × 10-4 moles of dichromate react with 3 × 7.51 × 10-4 mol = 2.25 × 10-3 moles ethanol
Remember the first step in the experiment when a 20.0 mL aliquote of diluted wine was placed in the conical flask?
This means that that 20.0 mL sample contained 2.25 × 10-3 moles ethanol.
- Calculate the concentration of ethanol in the conical flask at the start of the experiment:
concentration (mol L-1) = moles ÷ volume (L)
moles (C2H5OH) = 2.25 × 10-3 mol
volume (C2H5OH) = 20.0 mL = 20.0 mL ÷ 1000 mL/L = 0.020 L
concentration (C2H5OH) = 2.25 × 10-3 ÷ 0.020 = 0.11 mol L-1
- Calculate the concentration of ethanol in the wine
Remember that the original wine was diluted, 10.0 mL of wine was placed in a 250.0 mL volumetric flask and water was added up to the mark.
Then a 20.0 mL aliquot of this diluted wine was used in the titration.
We have so far calculated the concentration of ethanol in that 20.0 mL sample and found it to be 0.11 mol L-1
That means that the concentration of the diluted wine in the 250.0 mL volumetric flask was 0.11 mol L-1.
Let's calculate how many moles of ethanol must have been in that 250.0 mL volumetric flask:
concentration (mol L-1) = moles ÷ volume (L)
moles = concentration (mol L-1) × volume (L)
concentration (C2H5OH) = 0.11 mol L-1
volume = 250.0 mL = 250.0 ÷ 1000 mL/L = 0.250 L
moles (C2H5OH) = 0.11 × 0.250 = 0.028 mol
All 0.028 moles of ethanol must have come from the original 10.0 mL aliquot of wine that was added to the 250.0 mL volumetric flask.
Let's calculate the concentration of ethanol in that 10.0 mL wine sample (before it is diluted)
concentration (mol L-1) = moles ÷ volume (L)
moles (C2H5OH) = 0.028 mol
volume (wine) = 10.0 mL = 10.0 ÷ 1000 mL/L = 0.010 L
concentration (C2H5OH) = 0.028 ÷ 0.010 = 2.75 mol L-1
Since the 10.0 mL aliquot came directly from the wine to be tested, the concentration of ethanol in the wine must be 2.75 mol L-1
(If you go back to the beginning of the experiment you will remember that we said that the concentration of the wine will be 25 times the concentration of the diluted sample we use in the titration ... well .... 25 × 0.11 mol L-1 = 2.75 mol L-1!)
Calculation: Concentration of Ethanol in Wine as %(v/v)
Assume the density of ethanol is 0.79 g mL-1
- concentration of ethanol in wine was found to be 2.75 mol L-1 (see above)
This means that 1 L, or 1000 mL, of wine contains 2.75 mol of C2H5OH
- Calculate mass of ethanol in 1 L of wine:
moles = mass ÷ molar mass
mass (g) = moles × molar mass
moles (C2H5OH) = 2.75 mol
molar mass (C2H5OH) = (2 × 12.01) + (5 × 1.008) + 16.00 + 1.008 = 46.068 g mol-1
mass (C2H5OH) = 2.75 × 46.068 = 126.69 g
- Calculate the volume of 126.69 g of ethanol:
density = mass (g) ÷ volume (mL)
volume (mL) = mass (g) ÷ density (g mL-1)
density (C2H5OH) = 0.79 g mL-1
mass (C2H5OH) = 126.69 g
volume (C2H5OH) = 126.69 ÷ 0.79 = 160.37 mL
- Calculate the concentration of ethanol in wine in %(v/v)
% (v/v) = (volume of ethanol ÷ total wine volume) × 100
volume (C2H5OH) = 160.37 mL
volume (wine solution) = 1 L = 1000 mL
% (v/v) = (160.37 ÷ 1000) × 100 = 16.0 %
Quicker Calculation: Concentration of Ethanol in Wine
Once you understand the process that is occurring in this analysis, it is possible to take a couple of shortcuts in the calculations.
- Since all the reactions are taking place in the same vessel and nothing is leaving the system, we can add together all the iodine production equation and the titration equation:
iodine
production: |
Cr2O72-(aq) |
+ |
14H+ |
+ |
6I-(aq) |
→ |
2Cr3+(aq) |
+ |
7H2O(l) |
+ |
3I2(aq) |
| titration: |
|
|
3I2(aq) |
+ |
6S2O32-(aq) |
→ |
6I(aq) |
+ |
3S4O62-(aq) |
|
|
| |
|
overall
equation: |
Cr2O72-(aq) |
+ |
14H+ |
+ |
6S2O32-(aq) |
→ |
2Cr3+(aq) |
+ |
7H2O(l) |
+ |
3S4O62-(aq) |
Therefore moles of Cr2O72- not reacted with ethanol = 1/6 × moles S2O32-
moles of Cr2O72- not reacted with ethanol = 1/6 × (concentration S2O32- × volume (L))
- moles of Cr2O72- added to sample = concentration × volume (L)
So moles of Cr2O72- reacted with ethanol = (concentration Cr2O72- × volume Cr2O72-) - 1/6 × moles S2O32-
- moles of C2H5OH :
3C2H5OH(aq) + Cr2O72-(aq) + 8H+ → 3CH3CHO(aq) + 2Cr3+(aq) + 7H2O
moles of C2H5OH = 3 × moles Cr2O72- reacted
moles of C2H5OH = 3 × ((concentration Cr2O72- × volume Cr2O72-) - 1/6 × (concentration S2O32- × volume (L))
nC2H5OH = 3 × ((cCr2O72- × VCr2O72-) - 1/6 × (cS2O32- × VS2O32-)
concentration of ethanol in diluted sample = nC2H5OH ÷ volumeC2H5OH
concentration of ethanol in diluted sample = 3 × ((cCr2O72- × VCr2O72-) - 1/6 × (cS2O32- × VS2O32-)) ÷ volumeC2H5OH
concentration of ethanol in undiluted sample = dilution factor × (nC2H5OH ÷ volumeC2H5OH)
concentration of ethanol in undiluted sample = (final wine volume ÷ initial wine volume) × (3 × ((cCr2O72- × VCr2O72-) - 1/6 × (cS2O32- × VS2O32-)) ÷ volumeC2H5OH)
cethanol in wine = (Vfinal wine volume ÷ Vinitial wine volume) × (3 × ((cCr2O72- × VCr2O72-) - 1/6 × (cS2O32- × VS2O32-)) ÷ VC2H5OH)
Substituting in the values from the experiment above:
cethanol in wine = (Vfinal wine volume ÷ Vinitial wine volume) × (3 × ((cCr2O72- × VCr2O72-) - 1/6 × (cS2O32- × VS2O32-)) ÷ VC2H5OH)
cethanol in wine = (0.250 ÷ 0.010) x (3 x ((0.04 x 0.020) - 1/6 x (0.01 x 0.02962)) ÷ 0.020)
cethanol in wine = 25 x (3 x ((8.00 x 10-4 - 4.94 x 10-5) ÷ 0.020) = 2.81 mol L-1
Why isn't this answer EXACTLY the same as the one calculated above?
Because the step-by-step approach used above introduced more rounding errors into the calculation!
Experiment 2: Oxidation to Ethanoic (Acetic) Acid Method
This method gives good results for red wine, white wine, beer and spirits.
The concentration of ethanol (ethyl alcohol, C2H5OH) is to be determined using a redox back titration method.
Procedure:
- Dilute the alcoholic beverage:
Beer samples: pipette 20.0 mL beer into a 200.0 mL volumetric flask and make up to the mark with distilled water.
Wine samples: pipette 20.0 mL wine into a 1000.0 mL volumetric flask and make up to the mark with distilled water.
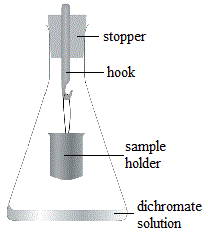
- Place 10.0 mL of acidified 0.010 mol L-1 potassium dichromate solution into a 250 mL conical flask2.
Pipette 1.0 mL of the diluted sample into the sample holder as shown in the diagram.
Place stoppered flask in an incubator at about 30°C over night.
- Remove flask from incubator and cool to room temperature.
Remove sample holder from flask.
Rinse walls of flask down into solution.
Add 100 mL of water to flask.
Add 1 mL of 1.2 mol L-1 potassium iodide solution3
- Titrate your sample flask with 0.030 mol L-1 sodium thiosulfate solution4.
Record your results.
Repeat the titration on different samples until concordant titres are achieved.
|
| Safety Considerations |
|---|
- Sulfuric acid is corrosive.
Wear safety glasses (goggles)
- Avoid contact with iodine.
Iodine stains skin, clothing & benches.
- Potassium dichromate is a carcinogen.
Avoid contact with skin.
|
|
Calculations:
- average titre (mL of Na2S2O3)
- moles of S2O32- used in titration with dichromate ions.
- moles of I2 present in the flask at the time of the titration.
- moles of Cr2O72- in the flask at the time of titration.
- moles of Cr2O72- added to the diluted wine sample at the start of the experiment.
- moles of Cr2O72- that reacted with ethanol.
- moles of C2H5OH in the diluted wine sample that reacted with Cr2O72-
- concentration of C2H5OH in diluted wine sample.
- concentration of C2H5OH in original, undiluted wine.
Calculation: Concentration of Ethanol in Wine in mol L-1
20.0 mL of wine was diluted to 1.0 L in a volumetric flask.
1 mL of diluted wine was suspended over 10.0 mL of acidified 0.010 mol L-1 potassium dichromate solution and left over night in an incubator at 30°C.
After cooling, 100 mL of water and 1 mL of 1.2 mol L-1 potassium iodide solution was added.
This solution was titrated with 0.030 mol L-1 sodium thiosulfate solution.
The experiment was repeated several times until concordant titres were acheived.
The average titre was calculated to be 14.50 mL of sodium thiosulfate solution.
Calculate the concentration of ethanol in the wine in mol L-1.
- Calculate the moles of S2O32- used in the titration:
concentration = 0.030 mol L-1
volume = 14.50 mL = 14.50 ÷ 1000 = 14.50 × 10-3 L
moles = concentration × volume = 0.030 × 14.50 x 10-3 = 4.35 × 10-4 moles
- Calculate moles of I2 that reacted with S2O32- in the titration:
Balanced chemical equation:
I2(aq) + 2S2O32-(aq) → 2I-(aq) + S4O62-(aq)
2 moles S2O32- react with 1 mole I2
1 mole S2O32- react with ½ moles I2
So, 4.35 × 10-4 moles S2O32- react with ½ × 4.35 × 10-4 = 2.18 × 10-4 moles I2
moles I2 = 2.18 × 10-4
- Calculate moles of Cr2O72- in flask:
Balanced redox equation:
Cr2O72-(aq) + 14H+ + 6I-(aq) → 2Cr3+(aq) + 7H2O(l) + 3I2(aq)
1 mole Cr2O72- produces 3 moles I2
1/3 mole Cr2O72- produces 1 mole I2
2.18 × 10-4 moles I2 is produced from 1/3 × 2.18 × 10-4 = 7.25 × 10-5 moles Cr2O72-.
moles Cr2O72- = 7.25 × 10-5 mol
- Calculate moles of dichromate that reacted with ethanol.
total moles Cr2O72- in flask = moles Cr2O72- reacted with ethanol + moles Cr2O72- in excess (reacted with I- to produce I2)
total moles Cr2O72- = concentration × volume added to flask originally
total moles Cr2O72- = 0.010 mol L-1 × 10.0/1000 = 1.00 × 10-4 mol
moles Cr2O72- in excess = 7.25 × 10-5 mol (see above)
moles Cr2O72- reacted with ethanol = total moles - moles reacted with I-
moles Cr2O72- reacted with ethanol = 1.00 × 10-4 - 7.25 × 10-5 = 2.75 × 10-5 mol
- Calculate moles of ethanol that reacted with Cr2O72-
Balanced redox equation :
3C2H5OH(aq) + 2Cr2O72-(aq) + 16H+ → 3CH3COOH(aq) + 4Cr3+(aq) + 11H2O
2 moles Cr2O72- react with 3 moles C2H5OH
1 mole Cr2O72- react with 3/2 moles C2H5OH
So, 2.75 × 10-5 moles of Cr2O72- react with 3/2 × 2.75 × 10-5 = 4.13 × 10-5 moles C2H5OH
moles C2H5OH = 4.13 × 10-5 mol
- Calculate concentration of ethanol in diluted wine sample:
moles = 4.13 × 10-5 mol
volume = 1 mL = 1 ÷ 1000 = 1.00 × 10-3 L
concentration = moles ÷ volume = 4.13 × 10-5 ÷ 1.00 × 10-3 = 0.0413 mol L-1
concentration of ethanol in diluted wine sample = 0.0413 mol L-1
- Calculate concentration of ethanol in undiluted wine:
ethanol concentration of diluted sample = 0.0413 mol L-1 (same as in the 1 mL aliquot calculated above)
volume of volumetric flask = 1.0 L
moles ethanol in volumetric flask = concentration × volume = 0.0413 × 1 = 0.0413 mol
All 0.0413 moles of ethanol must have been in the original 20.0 mL aliquot of wine added to the volumetric flask.
concentration of ethanol in wine = moles ÷ volume = 0.0413 ÷ 20.0 × 10-3 = 2.07 mol L-1
concentration of ethanol in wine = 2.07 mol L-1
Calculation: %(v/v) Concentration of Ethanol in Wine
- concentration of ethanol in wine was found to be 2.07 mol L-1 (see above)
This means that 1 L, or 1000 mL, of wine contains 2.07 mol of C2H5OH
- Calculate mass of ethanol in 1 L of wine:
moles = mass ÷ molar mass
mass (g) = moles × molar mass
moles (C2H5OH) = 2.07 mol
molar mass (C2H5OH) = (2 × 12.01) + (5 × 1.008) + 16.00 + 1.008 = 46.068 g mol-1
mass (C2H5OH) = 2.07 × 46.068 = 95.36 g
- Calculate the volume of 95.36 g of ethanol:
volume (mL) = mass (g) ÷ density (g mL-1)
density (C2H5OH) = 0.79 g mL-1
mass (C2H5OH) = 95.36 g
volume (C2H5OH) = 95.36 ÷ 0.79 = 120.7 mL
- Calculate the concentration of ethanol in wine in %(v/v)
% (v/v) = (volume of ethanol ÷ total wine volume) × 100
volume (C2H5OH) = 120.7 mL
volume (wine solution) = 1 L = 1000 mL
% (v/v) = (120.7 ÷ 1000) × 100 = 12.1 %
Quicker Calculation: Concentration of Ethanol in Wine
Once you understand the process that is occurring in this analysis, it is possible to take a couple of shortcuts in the calculations.
- Since all the reactions are taking place in the same vessel and nothing is leaving the system, we can add together all the iodine production equation and the titration equation:
iodine
production: |
Cr2O72-(aq) |
+ |
14H+ |
+ |
6I-(aq) |
→ |
2Cr3+(aq) |
+ |
7H2O(l) |
+ |
3I2(aq) |
| titration: |
|
|
3I2(aq) |
+ |
6S2O32-(aq) |
→ |
6I(aq) |
+ |
3S4O62-(aq) |
|
|
| |
|
overall
equation: |
Cr2O72-(aq) |
+ |
14H+ |
+ |
6S2O32-(aq) |
→ |
2Cr3+(aq) |
+ |
7H2O(l) |
+ |
3S4O62-(aq) |
Therefore moles of Cr2O72- not reacted with ethanol = 1/6 x moles S2O32-
moles of Cr2O72- not reacted with ethanol = 1/6 x (concentration S2O32- x volume (L))
- moles of Cr2O72- added to sample = concentration × volume (L)
So moles of Cr2O72- reacted with ethanol = (concentration Cr2O72- × volume Cr2O72-) - 1/6 × moles S2O32-
- moles of C2H5OH :
3C2H5OH(aq) + 2Cr2O72-(aq) + 16H+ → 3CH3COOH(aq) + 4Cr3+(aq) + 11H2O
moles of C2H5OH = 3/2 × moles Cr2O72- reacted
moles of C2H5OH = 3/2 × ((concentration Cr2O72- × volume Cr2O72-) - 1/6 × (concentration S2O32- × volume (L))
nC2H5OH = 3/2 × ((cCr2O72- × VCr2O72-) - 1/6 × (cS2O32- × VS2O32-)
concentration of ethanol in diluted sample = nC2H5OH ÷ volumeC2H5OH
concentration of ethanol in diluted sample = (3/2 × ((cCr2O72- × VCr2O72-) - 1/6 × (cS2O32- × VS2O32-))) ÷ volumeC2H5OH
concentration of ethanol in undiluted sample = dilution factor × (nC2H5OH ÷ volumeC2H5OH)
concentration of ethanol in undiluted sample = (final wine volume ÷ initial wine volume) × (3/2 × ((cCr2O72- × VCr2O72-) - 1/6 × (cS2O32- × VS2O32-))) ÷ volumeC2H5OH)
cethanol in wine = (Vfinal wine volume ÷ Vinitial wine volume) × (3/2 × ((cCr2O72- × VCr2O72-) - 1/6 × (cS2O32- × VS2O32-))) ÷ VC2H5OH)
Substituting in the values from the experiment above:
cethanol in wine = (Vfinal wine volume ÷ Vinitial wine volume) × (3/2 × ((cCr2O72- × VCr2O72-) - 1/6 × (cS2O32- × VS2O32-))) ÷ VC2H5OH)
cethanol in wine = (V1 L ÷ 0.020) × (3/2 × ((0.010 × 0.010) - 1/6 × (0.030 × 0.0145))) ÷ 0.001)
cethanol in wine = 50 × (3/2 × ((1.00 × 10-4 - 7.25 × 10-5) ÷ 0.001) = 2.06 mol L-1
Why isn't this answer EXACTLY the same as the one calculated above?
Because the step-by-step approach used above introduced more rounding errors into the calculation!
1. Other oxidising agents can be used, but potassium dichromate is a good primary standard because it can be obtained pure and is stable.
A primary standard solution of potassium dichromate can easily be prepared by weighing out the required mass and dissolving in enough water to make the required concentration.
The potassium dichromate solution can be stored indefinitely in a well-sealed vessel because the aqueous solution is stable.
By comparison, potassium permangante cannot be used as a primary standard because it cannot be obtained pure, it will readily react with traces of organic material or other reducing substances present in water, and decomposes in sunlight.
2. Prepare acidified dichromate solution by adding 70 mL concentrated sulfuric acid to 125 mL water in a 500 mL conical flask and adding 0.75 g potassium dichromate while cooling under tap water, then diluting to 250 mL with distilled water.
3. Prepare potassium iodide solution by dissolving 5 g of potassium iodide in 25 mL water.
4. Prepare 0.030 mol L-1 sodium thiosulfate solution by adding 7.44 g Na2S2O3.5H2O to a 1.0 L volumetric flask and making up to the mark with distilled water.


