Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Allotropes of Oxygen
There are two main allotropes of oxygen:
- A diatomic molecule made up of 2 oxygen atoms with the moelcular formula O2 commonly referred to as molecular oxygen or dioxygen.
- A triatomic molecule made up of 3 atoms of oxygen with the molecular formula O3 referred to as ozone.
Both allotropes of oxygen are made up only of oxygen atoms, but they differ in the arrangement of the oxygen atoms:
- Molecular oxygen (dioxygen), O2, is a linear molecule.
- Ozone, O3, is a bent molecule.
Molecular oxygen, O2, and ozone, O3, have different physical properties such as colour, odour, melting and boiling point, density and solubility.
Some properties of the allotropes of oxygen are shown in the table below:
| |
Allotropes of Oxygen |
|---|
| Property |
Molecular Oxygen (O2) |
Ozone (O3) |
|---|
| Structure |
O=O
linear |
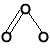
bent |
|
| Colour |
colourless gas
pale blue liquid
pale blue solid |
pale blue gas
deep blue liquid
deep violet solid |
|
| Odour |
odourless |
sharp, pungent |
|
| Melting Point (°C) |
-219 |
-193 |
|
| Boiling Point (°C) |
-183 |
-111 |
|
| Density (20°C) |
1.3 g L-1 |
2.0 g L-1 |
|
| Solubility in Water |
slightly soluble |
more soluble that O2 |
|
| Chemical Stability |
stable |
decomposes to O2 easily |
|
| Uses |
common oxidiser |
sterilising agent
(it is poisonous to many living things) |
Allotropes of Carbon
The two most common, naturally occurring allotropes of carbon:(1)
Both graphite and diamond are made up of carbon atoms, but the arrangement of atoms is different in each allotrope which results in different physical properties.
In particular, the presence of delocalised electrons in the structure of graphite results in it being soft and a good electrical conductor whereas diamond is very hard and an electrical insulator.
Some properties of graphite and diamond are shown in the table below:
| |
Allotropes of Carbon |
|---|
| Property |
Graphite |
Diamond |
|---|
| Structure |
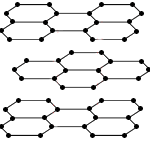 Each carbon atom is bonded to 3 other carbon atoms in layers with delocalised electrons between the layers. |
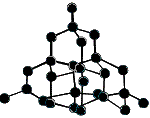 Each carbon atom is bonded to 4 other carbon atoms in a 3-dimensional covalent network. All valence electrons are used in bonding. |
|
| Colour |
black |
colourless |
|
| Melting Point (K) |
sublimes at ≈3500 |
sublimes at ≈4000 |
|
| Electrical Conductivity |
good
Delocalised electrons between the layers allow an electric current to pass through |
poor (an insulator)
No delocalised electrons to allow for the flow of electrical current |
|
| Hardness (Mohs Scale) |
1-2 (soft)
Delocalised electrons allow the sheets to move over each other |
10
Hardest known natural mineral. |
|
| Chemical Stability |
stable |
decomposes slowly over time |
|
| Uses |
lubricant
because it is soft |
abrasive
because it is so hard |
Allotropes of Phosphorus
There are three allotropes of phosphorus:
- white phosphorus
- red phosphorus
- black phosphorus
Some properties of the allotropes of phosphorus are given in the table below:
| Property |
White Phosphorus |
Red Phosphorus |
Black Phosphorus |
|---|
| Structure |
P4 molecules packed into a crystal |
Chains of P4 molecules
(polymer) |
Puckered layers of phosphorus atoms
(polymer) |
|
| Colour |
white |
red |
black |
|
| Chemical Stability |
least
stable |
intermediate
stability |
most
stable |
Allotropes of Sulfur
Sulfur has several allotropes:
- α-sulfur forms yellow, rhombic crystals out of 8-membered rings of sulfur atoms (S8).
- γ-sulfur forms yellow, monoclinic, needle-like crystals out of 8-membered rings of sulfur atoms (S8).
- Plastic sulfur is yellow and made up of long chains of sulfur atoms. It reverts to S8 rings in time.
Allotropes of Tin
There are three allotropes of tin:
- Grey tin (α tin): a diamond-type lattice structure
- White tin (β tin): body centred tetragonal structure
- brittle tin: rhombic structure



