Properties of Aspirin (acetylsalicylic acid)
Acidity
Aspirin is a monoprotic weak acid, Ka = 2.8 x 10-4 at 25oC, so very little of the molecular aspirin (acetylsalicylic acid) dissociates to form acetylsalicylate ions.
For the equilibrium dissociation reaction:
| aspirin (acetylsalicylic acid) |  | acetylsalicylate ion | + | H+ |
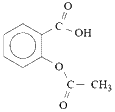 |  | 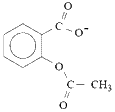 | + | H+ |
the equilibrium position lies well to the left, favouring molecular aspirin.
Solubility
Aspirin is only slightly soluble in water and acidic solutions such as is present in the stomach. Aspirin contains polar functional groups which can form hydrogen bonds with polar water molecules.
Aspirin is more soluble in basic (alkaline) solutions, so it readily dissolves in the duodenum which is the first part of the intestine.
Ionic salts of aspirin, such as sodium acetylsalicylate, are more soluble in water since they form stronger ion-dipole interactions with water.
These ionic salts of aspirin are sometimes marketed as "soluble aspirin". When you add water to the soluble aspirin, eg, sodium acetylsalicylate, it dissociates to form sodium ions and acetylsalicylate ions:
| sodium acetylsalicylate | → | acetylsalicylate ions | + | sodium ions |
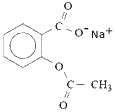 | → |  | + | Na+ |
| C9H7O4-Na+ | → | C9H7O4- | + | Na+ |
In the acidic stomach, molecular aspirin crystallizes out:
| acetylsalicylate ions | + | H+ | → | aspirin |
 | + | H+ | → |  |
| C9H7O4- | + | H+ | → | C9H8O4 |
Synthesis of Aspirin (acetylsalicylic acid)
Salicylic acid will rapidly react with acetic anhydride in the presence of an acid catalyst to produce aspirin (acetylsalicylic acid) and acetic acid (ethanoic acid).
Sulfuric acid or phosphoric acid are often used to catalyse the reaction.
| salicylic acid |
+ |
acetic anhydride
(ethanoic anhydride) |
[H2SO4]
→ |
aspirin
(acetylsalicylic acid) |
+ |
acetic acid
(ethanoic acid) |
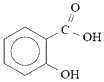 |
+ |
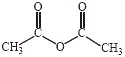 |
[H2SO4]
→ |
 |
+ |
CH3COOH |
| C7H6O3(s) |
+ |
C4H6O3(l) |
[H2SO4]
→ |
C9H8O4(s) |
+ |
C2H4O2(aq) |
Salicylic acid can react with acetic (ethanoic) acid in an esterification reaction, but the reaction is very slow, taking days to reach equilibrium, and the yield is low:
| salicylic acid |
+ |
acetic acid
(ethanoic acid) |
[H2SO4]
→ |
aspirin
(acetylsalicylic acid) |
+ |
water |
 |
+ |
CH3COOH |
[H2SO4]
→ |
 |
+ |
H2O |
| C7H6O3 |
+ |
C2H4O2 |
[H2SO4]
→ |
C9H8O4(s) |
+ |
H2O |
For this reason, the commercial preparation of aspirin relies on the faster reaction between salicylic acid and the more reactive acetic anhydride which produces a greater yield of aspirin.

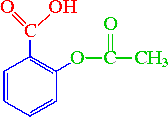 Aspirin (acetylsalicylic acid) contains three groups:
Aspirin (acetylsalicylic acid) contains three groups:





