Kinetic Theory of Gases Tutorial
Key Concepts
- The kinetic theory, or the kinetic-molecular theory, of gases explains the properties of ideal gases.
- The kinetic theory of gases is based on the following four assumptions:
1. A gas is composed of a large number of tiny particles which are so small that their sizes are negligible compared to the average distances between them, that is, most of the volume of a gas is just empty space.
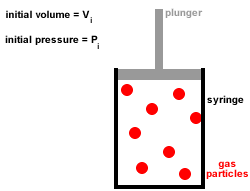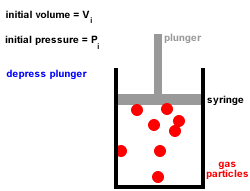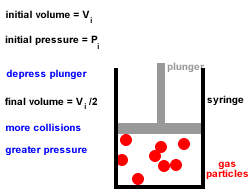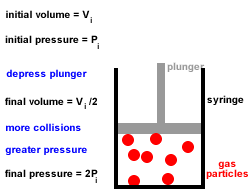
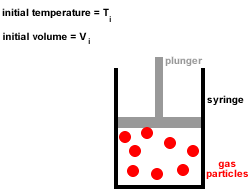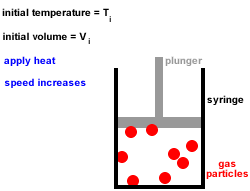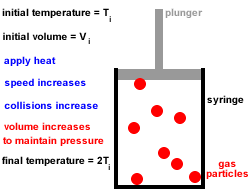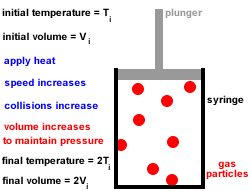
2. Gas particles are in constant, random, straight-line motion, colliding with each other and the walls of the container.
All collisions are elastic, there is no net loss or gain of kinetic energy at each collision.3. Gas particles move independently of each other, there are no forces of attraction or repulsion between them.
4. At any given instant there is a wide range of particle speeds and therefore a wide range of molecular kinetic energies, however, the average kinetic energy of all the gas particles is proportional to the absolute temperature (temperature in kelvin).
The kinetic energy, Ek or K.E, of a single gas particle is given by the equation below:
Ek = ½mv2
where m = mass of gas particle and v = speed of gas particle
- Gases will deviate from ideal gas behaviour if one or more of these assumptions does not apply