Measuring Reaction Rates
How a reaction rate is measured depends on the nature of the reactants and products.
Some measurable quanitities are:
- the volume of gas evolved per unit time
- the mass of solid formed per unit time
- the intensity of colour per unit time
- the change in pH per unit time
- the change in temperature per unit time
Imagine an experiment in which we can measure the amount of product being formed in a closed vessel.2
At the start of the experiment, only reactants are present, there are no products yet, so the amount of product is zero.
But after that, measurable amounts of product are produced, and we record the amount of product produced every minute (for example).
Then, imagine we repeat the experiment, keeping all the variables the same, EXCEPT that we heat the whole system.
When we plot the points of amount of product produced vs time, for the same reaction at two different temperatures, we get a graph like the one shown below:
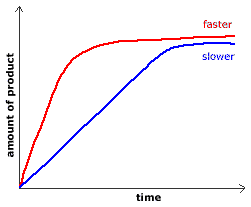 |
____ high temperature
____ low temperature
|
The initial slope of the graph provides you with the initial rate of reaction.
A fast reaction will have a steeper slope than a slower reaction.
On the graph above, the red line represents a faster initial reaction rate than the blue line because the slope of the red line is greater than the slope of the blue line in the early (initial) stages of the reaction.
Reaction rates slow down as the reaction approaches equilibrium.
As products are formed, there are fewer reactant particles to react which means there will be fewer successful collisions, so, the reaction rate decreases.
At equilibrium, the concentration of a reactant, or the concentration of a product, does not change with time.
At equilibrium each line in the graph above will be straight horizontal line.
Worked Example: Zinc and Hydrochloric Acid Reaction Rate
Consider an experiment in which we add hydrochloric acid, HCl(aq), to a clean piece of metallic zinc, Zn(s), in an open beaker.
The products of this reaction will be water soluble zinc chloride, ZnCl2(aq), and hydrogen gas, H2(g).
Because this is an "open system" in which the hydrogen gas can escape, we will use a single-headed arrow, →, to show the reaction goes to completion in one direction as shown in the balanced chemical reaction below:
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
In order to determine the rate of the reaction we could:
- Measure the rate at which a reactant disappears:
For example, we could remove the piece of zinc from the beaker every minute and weigh it separately, recording its mass before placing it back into the beaker and starting the timer again.
Over time, the mass of zinc would decrease.
- Measure the rate at which a product appears:
For example, we could use the "water displacement" method to collect the hydrogen gas and measure its volume every minute.
Over time, the volume of gas would increase.
We could then investigate the factors that influence the rate of reaction by changing one variable at a time and repeating the experiment.
For example, we could study the effect of changing the concentration of the acid on the reaction by rate by repeating the experiment several times using the same volume of acid BUT each time using a different concentration of acid, while keeping all other variables constant, that is,
- same volume of hydrochloric acid in all experiments
- same temperature for all experiments
- same atmospheric pressure for all experiments
- same clean, dry beaker for all experiments
- same mass of zinc for all experiments
- same dimensions (width, height and breadth) for the piece of zinc
(see Experimental Design Tutorial for more details of how to design your own experiments.)
If we were to perform a set of experiments to test each of the variables that we think would effect the rate at which zinc metal reacts with hydrochloric acid, we would find results such as those given in the table below:
| Variable |
Affect on Rate |
Explanation |
|---|
| Concentration |
Increasing the concentration of HCl(aq) will increase the reation rate. |
More HCl particles3 means there will be more collisions between HCl(aq) and Zn(s). |
| Temperature |
Increasing temperature increases the reaction rate. |
HCl particles will gain more kinetic energy increasing the number of collisions with Zn atoms.
More Zn(s) and HCl(aq) particles will have sufficient energy to react resulting in more successful collisions. |
| Particle Size |
Reducing the size of Zn(s) particles will increase the rate of reaction. |
Reducing the size of the Zn(s) particles increases the surface area available for reaction with HCl(aq) molecules resulting in more collisions. |
| Stirring Rate |
Increasing the stirring rate of this mixture will increase the reaction rate. |
Stirring will keep small Zn(s) particles in suspension, increasing the surface area available for collisions, resulting in an increased reaction rate. |
1. "Surface area to volume ratios" crop up all the time in chemistry. As the size of a particle (as measured by its radius or diameter) decreases, the amount of substance at the surface of the particle compared to the amount of substance occupying the body (or volume) of the particle increases dramatically.
See the Nanoparticles and Nanotechnology Tutorial for an indepth discussion of how this happens.
2. In a closed system our reaction can reach equilibrium, and at this point the concentrations of reactants and products that we measure will not change with time.
However, in an open system it might be possible for some of our reactants and/or products to escape from the system.
In this case the system we are studying will not reach equilibrium.
3. For the purposes of this discussion it doesn't matter whether you think of the "acid particles" as HCl(aq) particles or as H+(aq) or H3O+(aq) particles.
The point is that increasing the concentration of the acid increases the number of "acid particles" irrespective of their identity.

