Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Examples of Initial Concentration Method to Determine Rate Law
Question 1 : Determining the Rate Law of a Zero Order Reaction
Ammonia, NH3(g), can be decomposed on the surface of tungsten metal to produce nitrogen, N2(g), and hydrogen, H2(g), according to the equation:
2NH3(g) → 3H2(g) + N2(g)
The results of a series of experiments carried out at the same temperature are shown below:
| Trial |
Initial [NH3]
(M) |
Initial Rate
(M/min) |
|---|
| 1 |
2.50 × 10-3 |
6.67 × 10-7 |
| 2 |
5.00 × 10-3 |
6.67 × 10-7 |
Determine the rate law for this reaction.
Solution:
- Determine the change in initial concentration:
The initial concentration of NH3 was increased by a factor of
[NH3(trial 2)]
[NH3(trial 1)]
|
= |
5.00 × 10-3
2.50 × 10-3
|
= 2
|
- Determine the change in initial rate as a result of this change in concentration:
The initial rate of the reaction increased by a factor of
rate(trial 2)
rate(trial 1)
|
= |
6.67 × 10-7
6.67 × 10-7
|
= 1 |
That is, the rate does NOT change when the concentration of NH3 changes.
- Determine the relationship between the change in concentration and the change in rate:
Since increasing the concentration by a factor of 2 leads to no increase in the rate, the reaction rate is independent of the concentration of NH3
rate = k
The reaction is zero order.
- Calculate the specific rate constant, k, for the reaction:
k = rate
Using data from Trial 1:
k = 6.67 × 10-7
- Write the rate law for this reaction:
rate = 6.67 × 10-7
Question 2: Determining the Rate Law of a First Order Reaction
Azomethane, C2H6N2, decomposes to produce ethane, C2H6, and nitrogen, N2.
C2H6N2(g) → C2H6(g) + N2(g)
The results of a series of experiments carried out at the same temperature are shown below:
| Trial |
Initial [C2H6N2(g)]
(M) |
Initial Rate
(M/min) |
|---|
| 1 |
2.00 × 10-2 |
3.00 × 10-4 |
| 2 |
4.00 × 10-2 |
6.00 × 10-4 |
Determine the rate law for this reaction.
Solution:
- Determine the change in initial concentration:
The initial concentration of C2H6N2(g) was increased by a factor of
[C2H6N2(g)(trial 2)]
[C2H6N2(g)(trial 1)]
|
= |
4.00 × 10-2
2.00 × 10-2
|
= 2 |
- Determine the change in initial rate as a result of this change in concentration:
The initial rate of the reaction increased by a factor of
rate(trial 2)
rate(trial 1)
|
= |
6.00 × 10-4
3.00 × 10-4
|
= 2 |
- Determine the relationship between the change in concentration and the change in rate:
Since increasing the concentration by a factor of 2 leads to an increase in the rate by a factor of 2, the reaction rate and the concentration of C2H6N2(g) are directly proportional:
rate ∝ [C2H6N2(g)]
So, rate = k[C2H6N2(g)]
The reaction is first order.
- Calculate the specific rate constant, k, for the reaction:
Using data from Trial 1:
| k = |
3.00 × 10-4
2.00 × 10-2
|
= 0.015 |
- Write the rate law for this reaction:
rate = 0.015[C2H6N2(g)]
Question 3: Determining the Rate Law of a Second Order Reaction
Hydrogen iodide decomposes to hydrogen and iodine according to the equation:
2HI → H2 + I2
The results of a series of experiments carried out at the same temperature are shown below:
| Trial |
Initial [HI] (M) |
Intial Rate (M/min) |
|---|
| 1 |
1.50 × 10-3 |
1.20 × 10-5 |
| 2 |
3.00 × 10-3 |
4.8 × 10-5 |
Determine the rate law for this reaction.
Solution:
- Determine the change in initial concentration:
The initial concentration of HI was increased by a factor of
[HI2(g)(trial 2)]
[HI2(g)(trial 1)]
|
= |
3.00 × 10-3
1.50 × 10-3
|
= 2 |
- Determine the change in initial rate as a result of this change in concentration:
The initial rate of the reaction increased by a factor of
rate(trial 2)
rate(trial 1)
|
= |
4.8 × 10-5
1.20 × 10-5
|
= 4 |
= 22 |
- Determine the relationship between the change in concentration and the change in rate:
Since increasing the concentration of HI by a factor of 2 leads to an increase in the rate by a factor of 22, the rate is proportional to the square of the concentration of HI,
rate ∝ [HI]2
So, rate = k[HI]2
The reaction is second order.
- Calculate the specific rate constant, k, for the reaction:
k = rate/[HI]2
Using data from Trial 1:
| k = |
1.20 × 10-5
[1.50 × 10-3]2
|
= |
1.20 × 10-5
2.25 × 10-6
|
= 5.33 |
- Write the rate law for this reaction:
rate = 5.33[HI]2
Examples of Graphical Method for Determining Rate Law
Question 1: Determining the Rate Law of a First Order Reaction
The decomposition of gaseous dinitrogen pentoxide, N2O5, was investigated at a particular temperature.
The results of the experiment are shown below:
Time
(min) |
0.00 |
5.00 |
10.00 |
15.00 |
20.00 |
25.00 |
|---|
[N2O5]
(M) |
1.30 |
1.08 |
0.90 |
0.75 |
0.62 |
0.52 |
|---|
Show that the reaction is first order and determine the rate law for this reaction.
Solution:
- Calculate log10[N2O5]
Time
(min) |
0.00 |
5.00 |
10.00 |
15.00 |
20.00 |
25.00 |
|---|
[N2O5]
(M) |
1.30 |
1.08 |
0.90 |
0.75 |
0.62 |
0.52 |
|---|
| log10[N2O5] |
0.11 |
0.03 |
-0.05 |
-0.12 |
-0.21 |
-0.28 |
|---|
- Plot log10[N2O5] against time (shown below)
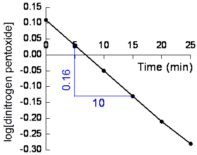
Straight line graph means the reaction is first order.
Rate is directly proportional to N2O5 concentration.
rate ∝ [N2O5]
rate = k[N2O5]
- Calculate the slope of the line:
from the graph (shown in blue):
| slope = |
-0.16
10.00 |
= -0.016 |
mathematically:
| slope = |
y1 - y2
x1 - x2 |
| = |
0.11 - (-0.28)
0.00 - 25.00 |
| = |
0.39
-25.00 |
| = |
-0.016 |
- Calculate the specific rate constant, k, for this reaction:
| slope |
= |
-k
2.303 |
| 2.303 × slope |
= |
-k |
| -2.303 × slope |
= |
k |
Substitute the value for the slope into the equation and solve to find the specific rate constant, k
k = -slope × 2.303
= -(-0.016) × 2.303
= 0.037
- Write the rate law for this reaction:
rate = 0.037[N2O5]
Question 2: Determining the Rate Law of a Second Order Reaction
An investigation into the decomposition of NO2 at a particular temperature produced the following results:
Time
(s) |
0.00 |
5.00 |
10.00 |
15.00 |
20.00 |
|---|
[NO2]
(M) |
0.10 |
0.017 |
0.0089 |
0.0062 |
0.0047 |
|---|
Use the data to verify that that reaction is second order and determine the rate law for the reaction.
Solution:
- Calculate the reciprocal concentrations of NO2
Time
(s) |
0.00 |
5.00 |
10.00 |
15.00 |
20.00 |
|---|
[NO2]
(M) |
0.10 |
0.017 |
0.0089 |
0.0062 |
0.0047 |
|---|
| 1/[NO2] |
10 |
59 |
112 |
161 |
213 |
|---|
- Plot 1/[NO2] against time (shown below):
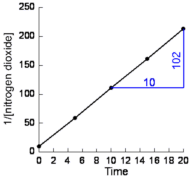
Straight line graph means the reaction is second order.
rate is proportional to the square of the concentration
rate ∝ [NO2]2
rate = k[NO2]2
- Calculate the slope of the line:
from the graph (shown in blue):
mathematically:
| slope = |
(y1 - y2)
(x1 - x2)
|
| = |
(10 - 213)
(0 - 20)
|
| = |
-203
-20 |
| = |
10.2 |
- Calculate the specific rate constant, k, for this reaction:
slope = k = 10.2
- Write the rate law for this reaction:
rate = 10.2[NO2]2
Examples of Half-Life Method for Determining Rate Law
Question 1: Using a Graph to Determine Half-Life
The results of the decomposition of gaseous dinitrogen pentoxide, N2O5, at various times are tabulated below.
Time
(min) |
0.00 |
10 |
20 |
30 |
40 |
50 |
60 |
|---|
[N2O5]
(M) |
348.8 |
247 |
185 |
140 |
105 |
78 |
58 |
|---|
Use the data to confirm that this reaction is first order and find the specific rate constant at this temperature.
Solution:
- Plot [N2O5] against time as shown below:

- Use the graph to obtain half-life values:
[N2O5]0
(M) |
Graph
Reference |
t½
(min) |
| 300 |
a to b |
24 |
| 250 |
c to d |
24 |
| 200 |
e to f |
24 |
| 150 |
b to g |
24 |
- Half-life is independent of [N2O5]0 so the reaction is first order.
That is, as [N2O5]0 changes, the half-life, t½ is constant
- Calculate the specific rate constant, k.
For a first order reaction:
| t½ |
= |
0.693
k |
| k |
= |
0.693
t½ |
| |
= |
0.693
24 |
| |
= |
0.029 |
Question 2: Using the Exponential Equation to Determine Half-Life
The decomposition of gaseous N2O3 to form NO2 and NO is first order with k = 3.2 × 10-4s-1.
If the initial concentration of N2O3 is 1.00 M, how long will it take for its concentration to be decreased to 0.125 M ?
Solution:
- The relationship between concentration and time is :
| log10[A]t |
= |
-kt
2.303 |
+ log10[A]0 |
- Extract the data from the question:
[N2O3]0 = 1.00 M
[N2O3]t = 0.125 M
k = 3.2 × 10-4s-1
t = ?
- Substitute the values into the equation:
| log10[A]t |
= |
-kt
2.303 |
+ log10[A]0 |
| log10[0.125] |
= |
-3.2 x 10-4t
2.303 |
+ log10[1.00] |
| -0.903 |
= |
-3.2 x 10-4t
2.303 |
+ 0 |
-0.903 × 2.303
-3.2 x 10-4 |
= |
t |
|
| 6499 s |
= |
t |
|
Alternatively:
| [N2O3]t |
= |
[N2O3]0e-kt |
ln( [N2O3]t )
[N2O3]0 |
= |
-kt |
ln([N2O3]t/[N2O3]0
-k |
= |
t |
ln(0.125/1.00)
-(-3.2 × 10-4) |
= |
t |
| 6498 s |
= |
t |
Alternatively:
- Recognize that the time for 1.00 M to reduce to 0.125 M is equivalent to 3 half-lives:
| Time |
[N2O3] |
|---|
| 0 |
1.00 |
| 1 half-life |
0.500 M |
| 2 half-lives |
0.250 M |
| 3 half-lives |
0.125 M |
- For a first order reaction:
| t½ |
= |
0.693
k |
| |
= |
0.693
3.2 × 10-4 |
| |
= |
2166 s |
- 3 half-lives = 3 × 2166 = 6498 s
1. If you are looking for information about radioactive half-life, go to the Half-life Tutorial



