s, p, d, and f Blocks of the Periodic Table of the Elements Chemistry Tutorial
Key Concepts
⚛ Electrons making up an atom are arranged in subshells.(1)
⚛ There are four types of subshells.
⚛ Each of the subshells is designated by a letter, either s, p, d or f:
s subshell, p subshell, d subshell, and f subshell
⚛ By considering which type of subshell is being filled with electrons we can see a pattern in the Periodic Table of the Elements:
s subshell: Group 1 , Group 2, hydrogen and helium
d subshell: Groups 3 to 12 (transition metals)
p subshell: Groups 13 to 18
f subshell: lanthanoids and actinoids(2)
Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Location of s, p, d, and f Blocks in the Periodic Table
The Periodic Table below probably looks a little bit different to the one you are used to seeing.
We have separated hydrogen (H) and helium (He) from the other main group elements.
Notice the location of the labels:
s block: first 2 columns on the left hand side of the Periodic Table
d block: 10 columns in the middle of the Periodic Table
p block: last 6 columns on the right hand side of the Periodic Table
f block: bottom 2 rows separated from the rest of the Periodic Table
| Period 1 |
1
H
hydrogen
1.008
|
2
He
helium
4.003 |
|
| |
Group 1 |
Group 2 |
Group 3 |
Group 4 |
Group 5 |
Group 6 |
Group 7 |
Group 8 |
Group 9 |
Group 10 |
Group 11 |
Group 12 |
Group 13 |
Group 14 |
Group 15 |
Group 16 |
Group 17 |
Group 18 |
| |
s block |
d block |
p block |
| Period 2 |
3
Li
lithium
6.941 |
4
Be
beryllium
9.012 |
|
5
B
boron
10.81 |
6
C
carbon
12.01 |
7
N
nitrogen
14.01 |
8
O
oxygen
16.00 |
9
F
fluorine
19.00 |
10
Ne
neon
20.18 |
| Period 3 |
11
Na
sodium
22.99 |
12
Mg
magnesium
24.31 |
|
13
Al
aluminium
26.98 |
14
Si
silicon
28.09 |
15
P
phosphorus
30.97 |
16
S
sulfur
32.07 |
17
Cl
chlorine
35.45 |
18
Ar
argon
39.95 |
| Period 4 |
19
K
potassium
39.10 |
20
Ca
calcium
40.08 |
21
Sc
scandium
44.96 |
22
Ti
titanium
47.87 |
23
V
vanadium
50.94 |
24
Cr
chromium
52.00 |
25
Mn
manganese
54.94 |
26
Fe
iron
55.85 |
27
Co
cobalt
58.93 |
28
Ni
nickel
58.69 |
29
Cu
copper
63.55 |
30
Zn
zinc
65.41 |
31
Ga
gallium
69.72 |
32
Ge
germanium
72.64 |
33
As
arsenic
74.92 |
34
Se
selenium
78.96 |
35
Br
bromine
79.90 |
36
Kr
krypton
83.80 |
| Period 5 |
37
Rb
rubidium
85.47 |
38
Sr
strontium
87.62 |
39
Y
yttrium
88.91 |
40
Zr
zirconium
91.22 |
41
Nb
niobium
92.91 |
42
Mo
molybdenum
95.94 |
43
Tc
technetium
[97.91] |
44
Ru
ruthenium
101.1 |
45
Rh
rhodium
102.9 |
46
Pd
palladium
106.4 |
47
Ag
silver
107.9 |
48
Cd
cadmium
112.4 |
49
In
indium
114.8 |
50
Sn
tin
118.7 |
51
Sb
antimony
121.8 |
52
Te
tellurium
127.6 |
53
I
iodine
126.9 |
54
Xe
xenon
131.3 |
| Period 6 |
55
Cs
caesium
132.9 |
56
Ba
barium
137.3 |
57
La
lanthanum
138.9 |
72
Hf
hafnium
178.5 |
73
Ta
tantalum
180.9 |
74
W
tungsten
183.8 |
75
Re
rhenium
186.2 |
76
Os
osmium
190.2 |
77
Ir
iridium
192.2 |
78
Pt
platinum
195.1 |
79
Au
gold
197.0 |
80
Hg
mercury
200.6 |
81
Tl
thallium
204.4 |
82
Pb
lead
207.2 |
83
Bi
bismuth
209.0 |
84
Po
polonium
[209.0] |
85
At
astatine
[210.0] |
86
Rn
radon
[222.0] |
| Period 7 |
87
Fr
francium
[223.0] |
88
Ra
radium
[226.0] |
89
Ac
actinium
[227.0] |
104
Rf
rutherfordium
[261.1] |
105
Db
dubnium
[262.1] |
106
Sg
seaborgium
[266.1] |
107
Bh
bohrium
[264.1] |
108
Hs
hassium
[277] |
109
Mt
meitnerium
[268] |
110
Ds
darmstadtium
[271] |
111
Rg
roentgenium
[272] |
112
Cn
copernicium
|
113
Nh
nihonium
|
114
Fl
flerovium
|
115
Mc
moscovium
|
116
Lv
livermorium
|
117
Ts
tennessine
|
118
Og
oganesson
|
| |
| f block |
Lanthanoids |
58
Ce
cerium
140.1 |
59
Pr
praseodymium
140.9 |
60
Nd
neodymium
144.2 |
61
Pm
promethium
[144.9] |
62
Sm
samarium
150.4 |
63
Eu
europium
152.0 |
64
Gd
gadolinium
157.3 |
65
Tb
terbium
158.9 |
66
Dy
dysprosium
162.5 |
67
Ho
holmium
164.9 |
68
Er
erbium
167.3 |
69
Tm
thulium
168.9 |
70
Yb
ytterbium
173.0 |
71
Lu
lutetium
175.0 |
| Actinoids |
90
Th
thorium
232.0 |
91
Pa
protactinium
231.0 |
92
U
uranium
238.0 |
93
Np
neptunium
[237.0] |
94
Pu
plutonium
[244.1] |
95
Am
americium
[243.1] |
96
Cm
curium
[247.1] |
97
Bk
berkelium
[247.1] |
98
Cf
californium
[251.1] |
99
Es
einsteinium
[252.1] |
100
Fm
fermium
[257.1] |
101
Md
mendelevium
[258.1] |
102
No
nobelium
[259.1] |
103
Lr
lawrencium
[262.1] |
|
↪ Back to top
Relationship between s ,p, d, and f Blocks and the Periodic Table Groups
There is a distinct pattern to the location of the s, p, d and f blocks, which is directly related to the Groups of the Periodic Table:
| Block |
Groups |
|---|
| s block |
Group 1 (alkali metals) and Group 2 (alkaline earth metals)
plus Period 1 (hydrogen and helium) |
| d block |
Groups 3 to 12 (transition metals) |
| p block |
Groups 13 to 18
(includes all Noble Gases, halogens and chalcogens) |
| f block |
Lanthanoids and Actinoids |
↪ Back to top
Relationship between s ,p, d, and f Blocks and Electronic Configuration
The labels s, p, d and f blocks of the Periodic Table refer to the subshell that is being filled with electrons.
⚛ Group 1 elements occur at the beginning of a new row (Period) of the Periodic Table.
The highest energy level (valence shell) contains only 1 electron in an s subshell.
⚛ Group 2 elements occur directly to the right of Group 1 elements.
The highest energy level (valence shell) contains 2 electrons, both electrons occupy an s subshell.
The s subshell for this energy level (shell) is now full.
⚛ The highest energy level (valence shell) of a Group 13 element already has 2 electrons in an s subshell, so the next electron occupies a p subshell to make 3 valence electrons in total (2 s electrons + 1 p electron).
As you proceed from left to right across the Period from Group 13 to Group 18 elements, electrons are being added to the p subshell.
Group 18 elements have 2 s electrons and 6 p electrons in their highest energy level (shell) which completes the s and p subshell.
⚛ Transition metals are filling their d subshell with electrons, starting with Group 3 elements which have 1 electron in a d subshell.
Group 12 elements have 10 electrons in a d subshell, which corresponds to a completed d subshell.
⚛ Lanthanoids and actinoids are filling their f subshells with electrons.
↪ Back to top
Sample Question
In the section of the periodic table shown below, the letters K, L, M and N have replaced the chemical symbols of four of the elements.
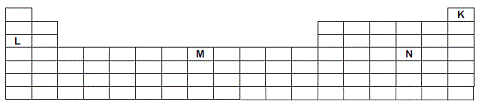
Identify the element which belongs to the d block of the periodic table.
↪ Back to top
Footnotes:
(1) Each subshell is made up of a set of orbitals, the orbitals reflect which subshell they belong to by using the same letter, that is, there are s orbitals, p orbitals, d orbitals and f orbitals.
However, although there is only one s orbital in the s subshell, there are 3 p orbitals in the p subshell, 5 d orbitals in the d subshell, and 7 f orbitals in the 5 subshell.
So, for the purposes of this discussion we will refer to s subshells, p subshells, d subshells and f subshells rather than to orbitals.
(2) Lanthanoids are also referred to as lanthanides, and actinoids are also referred to as actinides.
Strictly speaking the lanthanoids are the 14 elements following lanthanum (La) in the Periodic Table of the Elements, but since the term "lanthanoid" is used to indicate that these elements form a closely related group of which lanthanum is the prototype, the term is usually also applied to lanthanum itself.
In this discussion, we use the term lanthanoid in its strictest sense as meaning only the 14 elements immediatedly following lanthanum in the periodic table.
↪ Back to top

