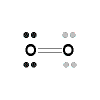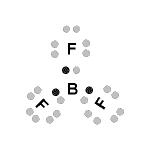Lewis Structures (electron dot diagrams) Chemistry Tutorial
Key Concepts
- Lewis structures are also known as:
⚛ electron dot diagrams
⚛ electron dot structures
⚛ Lewis dot diagrams
⚛ Lewis dot structures
⚛ Lewis electron dot diagrams
⚛ Lewis electron dot structures
- A dot is used to represent a valence electron.
Valence electrons occupy the highest energy level (also known as the valence shell).
- The chemical symbol of an element is surrounded by a number of dots.
The number of dots corresponds to the number of valence electrons.
- Lewis structures of Ionic compounds:
(i) Square brackets enclose the symbol of the element and the dots, the charge on the ion is shown as a superscript.
(ii) Lewis structures of ions of opposite charge are positioned next to each other to indicate an ionic compound.
- Lewis structures of covalent compounds:
(i) A single covalent bond between two atoms is indicated by one pair of electrons between the symbols for the elements.
(ii) A double covalent bond is represented by two pairs of electrons between adjacent symbols of atoms.
(iii) A triple covalent bond is represented by three paris of electrons between adjacent symbols of atoms.
- Some covalent compounds obey the octet rule by achieving a share of 8 electrons in their highest energy level (valence shell).
Many covalent compounds of Period 2 elements obey the octet rule.
- Many covalent compounds do not obey the octet rule:
(i) Hydrogen (Period 1) can only have 2 electrons in its highest energy level (K shell).
(ii) Atoms of elements with insufficient electrons to share to make up an octet of valence electrons can violate the octet rule.
(iii) Compounds in which there is an odd number of valence electrons violate the octet rule.
(iv) If the central atom in the compound is not a Period 2 element, it is possible for the octet rule to be violated for the central atom.
Please do not block ads on this website.
No ads = no money for us = no free stuff for you!
Lewis Structures of Atoms
The chemical symbol for the atom is surrounded by a number of dots corresponding to the number of valence electrons.
One dot . represents one valence electron.
For the main group elements1, the valence electrons are the electrons in the highest energy level (valence shell).
Hydrogen, symbol H, has 1 valence electron, Lewis structure is H.
Helium, symbol He, has 2 valence electrons, Lewis structure is He:
With helium, the first energy level (K shell) is full.
Lithium which has 3 electrons in total, has 2 electrons in the first energy level (K shell) just like helium, but these two electrons are not available for bonding so they are not valence electrons.
The third electron occupies the next energy level (L shell), which is a higher energy level than the first, and this electron is available to make bonds, so this electron is the valence electron.
An atom of lithium has only one valence electron so its Lewis structure is Li.
For the main group elements in the periodic table, there is a pattern to the number of valence electrons:
| Valence Electrons of Main Group Elements |
|---|
| |
Group 1
(Group IA) |
Group 2
(Group IIA) |
Group 13
(Group IIIA) |
Group 14
(Group IVA) |
Group 15
(Group VA) |
Group 16
(Group VIA) |
Group 17
(Group VIIA) |
Group 18
(Group VIIIA) |
|---|
No. valence
electrons |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|---|
Since each valence electron is represented by a single dot in the Lewis structure, there will also be a pattern to Lewis structures for atoms of the main group elements:
| Number of Dots in Lewis Structures of Main Group Elements |
|---|
| |
Group 1
(Group IA) |
Group 2
(Group IIA) |
Group 13
(Group IIIA) |
Group 14
(Group IVA) |
Group 15
(Group VA) |
Group 16
(Group VIA) |
Group 17
(Group VIIA) |
Group 18
(Group VIIIA) |
|---|
No. valence
electrons |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|---|
No. dots in
Lewis Structure |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|---|
So, if we let X represent the symbol of an atom of an element, we can write a general Lewis structure for each of the main group elements:
| General Lewis Structures of Main Group Elements |
|---|
| |
Group 1
(Group IA) |
Group 2
(Group IIA) |
Group 13
(Group IIIA) |
Group 14
(Group IVA) |
Group 15
(Group VA) |
Group 16
(Group VIA) |
Group 17
(Group VIIA) |
Group 18
(Group VIIIA) |
|---|
No. valence
electrons |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|---|
No. dots in
Lewis Structure |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|---|
General Lewis
Structure for
element X |
|
|
|
|
|
|
|
|
|---|
In order to write the Lewis structure for an atom of a main group element, just replace the X with the symbol for the element.
The table below shows the Lewis Structures for elements with atomic numbers 3 to 10 in the periodic table:
| Group |
1
(IA) |
2
(IIA) |
13
(IIIA) |
14
(IVA) |
15
(VA) |
16
(VIA) |
17
(VIIA) |
18
(VIIIA) |
|---|
| Number of Valence Electrons |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|---|
| Period 2 Example |
lithium |
beryllium |
boron |
carbon |
nitrogen |
oxygen |
fluorine |
neon |
|---|
Lewis Structure Example
(electron dot diagram) |
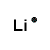 |
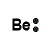 |
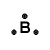 |
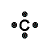 |
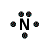 |
 |
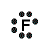 |
 |
|---|
Lewis Structures of Monatomic Ions
The chemical symbol for the element is surrounded by the number of valence electrons present in the ion.
The whole structure is then placed within square brackets, with a superscript to indicate the charge on the ion.
An atom will form an ion in order to achieve the same electron configuration as a Noble Gas (Group 18 element).
Group 18 (Noble Gas) elements will not form ions.
Negative ions (anions) are formed when an atom gains electrons.
An atom of hydrogen, with 1 valence electron, can gain 1 electron to form a hydrogen ion with a charge of -1 (the hydride ion) which has the same electron configuration as the Nobel Gas helium.
The Lewis structure for the hydride ion is [H:]-
For the main group atoms, an atom with many valence electrons (more than 4) will gain enough electrons to form a negative ion that has 8 valence electrons.
Positive ions (cations) are formed when an atom loses electrons.
An atom of hydrogen, with 1 valence electron, can lose this electron to form a hydrogen ion with a charge of +1 (a proton2).
The Lewis structure for this hydrogen ion is [H]+
For the main group atoms, an atom with few valence electrons (less than 4) will lose those valence electrons to form an ion with a positive charge equal to the number of electrons lost.
The table below summarises the Lewis Structures for the ions of the elements with atomic number 3 to 7
| Group |
1 |
2 |
13 |
14 |
15 |
16 |
17 |
|---|
| No. valence electrons in atom |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
|---|
| Atom forms ion by |
losing 1 electron |
losing 2 electrons |
losing 3 electrons |
losing 4 electrons |
gaining 4 electrons |
gaining 3 electrons |
gaining 2 electrons |
gaining 1 electron |
|---|
| Charge on Ion |
1+ |
2+ |
3+ |
4+ |
4- |
3- |
2- |
1- |
|---|
| Period 2 Element |
lithium |
beryllium |
boron |
carbon |
nitrogen |
oxygen |
fluorine |
|---|
| Li → Li+ + e- |
Be → Be2+ + 2e- |
B → B3+ + 3e- |
C → C4+ + 4e- |
C + 4e- → C4- |
N + 3e- → N3- |
O + 2e- → O2- |
F + e- → F- |
Lewis Structure
(electron dot diagram) |
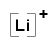
OR Li+ |
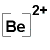
OR Be2+ |
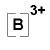 OR B3+ OR B3+ |
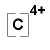
OR C4+ |
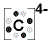 |
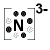 |
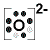 |
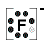 |
|---|
Lewis Structure of Ionic Compounds
The overall charge on the compound must equal zero, that is, the number of electrons lost by one atom must equal the number of electrons gained by the other atom.
The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound.
The Lewis structure of a positive ion (cation) is positioned adjacent to the Lewis structure of a negative ion (anion).
- If the charge on the positive ion is greater than the charge on the negative ion, draw another Lewis structure for another negative ion on the opposite side of the positive ion to the original negative charge (that is, do not position the Lewis structures for the two negative ions next to each other).
- If the charge on the positive ion is less than the charge on the negative ion, draw another Lewis structure for another positive ion on the opposite side of the negative ion to the original positive charge (that is, do not position the Lewis structures for the two positive ions next to each other).
Example: Lewis Structure for lithium fluoride, LiF
- Lithium atom (Group 1, 1 valence electron) loses one electron to form the cation Li+, [Li]+
- Fluorine atom (Group 17, 7 valence electrons) gains one electron to form the anion F-,
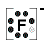
- Lithium fluoride compound is made up of 1 lithium ion and 1 fluoride ion and can be represented as
Li+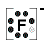 OR
OR 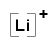
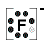
Example: Lewis Structure for lithium oxide, Li2O
- Each of the two lithium atoms (Group 1, 1 valence electron) loses one electron to form 2 cations Li+, [Li]+ (2 electrons in total are lost)
- Oxygen atom (Group 16, 6 valence electrons) gains two electrons to form the anion O2-,
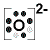
- Lithium oxide compound can be represented as:
2Li+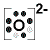 OR Li+
OR Li+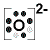 Li+ OR
Li+ OR 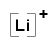
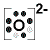
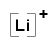
Lewis Structures for Covalent Compounds that Obey the Octet Rule
- In a covalent compound, electrons are shared between atoms to form a covalent bond in order that each atom in the compound has a share in the number of electrons required to provide a stable, Noble Gas, electronic configuration.
- Electrons in the Lewis Structure (electron dot diagram) are paired to show the bonding pair of electrons.
- Often this shared pair of electrons forming the covalent bond is circled
- Sometimes the bond itself is shown as a dash between atoms (-), these structures can be referred to as valence structures when all the lone (non-bonding) pairs of electrons are also shown.
- In order to complete its K shell, hydrogen (Period 1) needs a share in 2 electrons in order to achieve the same electronic configuration as the Noble gas atom helium.
Note that helium itself does not form covalent bonds as a result of the helium atom having a full valence shell of electrons.
- Period 2 elements (lithium to fluorine) need a share in 8 electrons to achieve the electron configuration of the Noble Gas (Group 18) element neon.
Note that neon itself does not form covalent bonds as a result of the neon atom having a full valence shell of electrons.
- The octet rule states that atoms have a tendency to complete the octet of electrons, that is, to achieve a Noble Gas (Group 18) electron configuration.
Example: Lewis Structure for hydrogen fluoride, HF
- Hydrogen atom has 1 valence electron
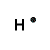
Hydrogen atom needs one more electron to complete its valence shell, that is, to make 2 electrons in its K shell.
- Fluorine atom (Group 17) has 7 valence electrons
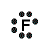
Fluorine atom needs one more electron to complete its valence shell, that is, to make 8 electrons in the L shell.
- Hydrogen will share its electron with fluorine to form a bonding pair of electrons (covalent bond) so that the hydrogen atom has a share in 2 valence electrons (electronic configuration of helium) and fluorine has a share in 8 valence electrons (electronic configuration of neon)
- Lewis Structure (electron dot diagram) for hydrogen fluoride
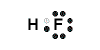 OR
OR 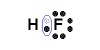
- The 2 electrons making up the bonding pair of electrons between the hydrogen atom and the fluorine atom, which may or may not be circled, are referred to as a covalent bond (or a single covalent bond).
- Note that the hydrogen atom has no lone pairs (non-bonding pairs) of electrons, but that the fluorine atom has 3 lone pairs (non-bonding pairs) of electrons.
- In the Valence Structure for hydrogen fluoride, the bonding pair of electrons (the covalent bond) is replaced with a dash (-) between the atoms:
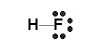
Note that the lone pairs (non-bonding) pairs of electrons are still shown around the fluorine atom in the valence structure.
Example: Lewis Structure for ammonia, NH3
- Nitrogen atom (Group 15) has 5 valence electrons
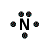
Nitrogen atom needs 3 more electrons in order to complete its valence shell, that is, to make up 8 electrons in the L shell.
- Hydrogen atom has 1 valence electron
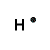
Hydrogen atom needs one more electron to complete its valence shell, that is, to make 2 electrons in its K shell.
- Each of the 3 hydrogen atoms in NH3 will share its electron with the central nitrogen atom to form a bonding pair of electrons (covalent bond) so that each hydrogen atom has a share in 2 valence electrons (electronic configuration of helium) and the nitrogen atom has a share in 8 valence electrons (electron configuration of neon)
- Lewis Structure (electron dot diagram) for ammonia
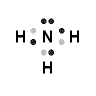 OR
OR 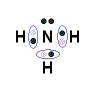
- Note that there are 3 covalent bonds (3 bonding pairs of electrons) in total, and that there is a lone pair (non-bonding pair) of electrons on the nitrogen atom.
- In the Valence Structure for ammonia, the bonding pairs of electrons, which may or may not be circled in the Lewis structure, are replaced by a dash (-) between atoms to represent the covalent bond:
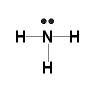
Example: Lewis Structure for oxygen molecule, O2
- Each oxygen atom (Group 16) has 6 valence electrons

An oxygen atom needs 2 more electrons in order to complete its valence shell, that is, to make up 8 electrons in the L shell.
- Each oxygen atom will share 2 of its valence electrons in order to form 2 bonding pairs of electrons (a double covalent bond) so that each oxygen atom will have a share in 8 valence electrons (electronic configuration of neon).
- Lewis Structure (electron dot diagram) for the oxygen molecule, O2,
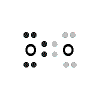 OR
OR 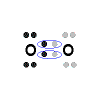
- There are 2 bonding pairs of electrons shared between the 2 oxygen atoms, and each oxygen atom also has 2 lone pairs (non-bonding) pairs of electrons.
- In the Valence structure for the oxygen molecule, each bonding pair of electrons is replaced by a dash (-) to represent a covalent bond:
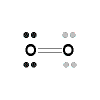
Note that there are 2 dashes, 2 covalent bonds, joining one oxygen atom to the other, this is referred to as a double covalent bond (or covalent double bond, or just as a double bond).
Example: Lewis Structure for nitrogen molecule, N2
- Each nitrogen atom (Group 15) has 5 valence electrons
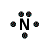
A nitrogen atom needs 3 more electrons in order to complete its valence shell, that is, to make up 8 electrons in the L shell.
- Each nitrogen atom will share 3 of its valence electrons in order to form 3 bonding pairs of electrons (a triple covalent bond) so that each nitrogen atom will have a share in 8 valence electrons (electronic configuration of neon).
- Lewis Structure (electron dot diagram) for the nitrogen molecule, N2,
- There are 3 bonding pairs of electrons shared between the 2 nitrogen atoms, and each nitrogen atom also has 1 lone pair (non-bonding) pair of electrons.
- In the Valence structure for the nitrogen molecule, each bonding pair of electrons is replaced by a dash (-) to represent a covalent bond:
:N≡N:
Note that there are 3 dashes, 3 covalent bonds, joining one nitrogen atom to the other, this is referred to as a triple covalent bond (or covalent triple bond, or just as a triple bond).
Lewis Structures for Other Covalent Compounds that DO NOT Obey the Octet Rule
Many covalent compounds do not obey the octet rule.
This may be due to:
- insufficient valence electrons available to make an octet
- an odd number of valence electrons
- an expanded valence shell (that is, availability of d orbitals from Period 3 onwards3)
Example: Insuffcient valence electrons to make an octet
- Consider the molecule BF3
- An atom of boron (Period 2, Group 13) has 3 valence electrons:
A boron atom would require a share in 5 more electrons to complete its octet of valence electrons.
- An atom of fluorine (Period 2, Group 17) has 7 valence electrons:
Each fluorine atom needs a share in one more electron to complete its octet of valence electrons
- So each fluorine atom will provide 1 electron to be shared with the boron atom, and the boron atom will provide 1 electron to be shared with each fluorine atom, resulting in the following Lewis structure for BF3:
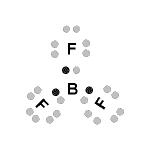
- Note that each fluorine atom has completed its octet of electrons, but that the boron atom has a share in only 6 electrons, that is, less than an octet of electrons.
There are insufficient valence electrons available for boron to achieve a Noble gas (Group 18) electron configuration.
Example: Odd number of valence electrons
- Consider the molecule NO
- An atom of nitrogen (Period 2, Group 15) has 5 valence electrons:
- An atom of oxygen (Period 2, Group 16) has 6 valence electrons:
- The total number of valence electrons is 5 + 6 = 11 = an odd number so it will be impossible to make a molecule of NO in which all the electrons will be paired, there will always be one electron without a partner.
- A nitrogen atom requires a share in 3 more electrons to complete its octet of valence electrons.
An oxygen atom requires a share in 2 more electrons in order to complete its octet of valence electrons
- So, in order for oxygen to complete its octet, the nitrogen atom will provide 2 electrons to share and the oxygen atom will also provide 2 electrons to share:
A double covalent bond forms between the nitrogen and oxygen atoms, the oxygen atom has completed its octet of valence electrons but the nitrogen atom only has a share in 7 valence electrons, that is, it is 1 electron short of completing its octet4.
Example: Expanded valence shell
- Consider the molecule PCl5
- An atom of phosphorus (Period 3, Group 15) has 5 valence electrons:
A phosphorus atom requires a share in 3 more electrons to complete its octet of valence electrons.
- An atom of chlorine (Period 3, Group 17) has 7 valence electrons:
- In order to form the compound PCl5, each chlorine atom must share one of its electrons with the central phosphorus atom, and the phosphorus atom must share 1 of its 5 electrons with each of the chlorine atoms:

Each chlorine atom has completed its octet of electrons, but the central phosphorus atom has a share in 10 valence electrons which is 2 more than an octet of electrons.
The valence shell of the phosphorus atom is said to have expanded in order to accommodate all 5 bonding pairs of electrons.
This is possible because there are d orbitals available to the third energy level, that is, 3d orbitals.
The phosphorus atom in the PCl5 molecule can use a 3d orbital, as well as the 3s and 3p orbitals, to house the required bonding pairs of electrons.
- Note that the valence shell of Period 1 and Period 2 elements cannot be expanded because d orbitals are not available in the first and second energy levels.
Footnotes
1. Main group elements are Group 1 (IA or alkali metals), Group 2 (IIA or alkaline earth metals), Group 13 (IIIA), Group 14 (IVa), Group 15 (VA), Group 16 (VIA or chalcogens), Group 17 (VIIA or halogens) and Group 18 (0 or VIIIA, or Noble Gases or Inert Gases).
The following discussion will not refer to transition metals, lanthanoids (lanthanides) or actinoids (actinides).
2. International Union of Pure and Applied Chemistry (IUPAC) prefers the term hydron to proton, but you are probably more likely to see, and use, the term proton.
99.99% of naturally occurring hydrogen atoms on earth are made up of 1 proton in the nucleus (zero neutrons) and 1 valence electron (the protium atom), so losing the electron results in a "naked" proton.
Only 0.01% of naturally occurring hydrogen atoms are made up of a nucleus containing 1 proton and 1 neutron and 1 valence electron (the deuterium atom), so losing the electron results in a nucleus containing 1 proton and 1 neutron (the deuteron ion).
The term hydron therefore refers to positively charged ions of the naturally occuring isotopic mix of hydrogen atoms.
3. The d orbitals are not required when writing the electron configuration of atoms of Period 3 elements, only s and p orbitals are needed.
However, the valence shell is the third energy level (M shell), which means that the 3d orbitals are available to these atoms along with the 3s and 3p orbitals if required when making covalent bonds.
In period 4, the transition metals fill the 3d orbitals, and Groups 13 to 18 fill the 4p orbitals, but now the 4d orbitals will be available if required for bonding purposes.
4. While this is an acceptable Lewis Structure for nitric oxide, NO, it is not really a very satisfactory desciption oif the molecule. Resonance theory or Molecular Orbital (MO) theory give more satisfactory descriptions in this instance.

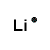
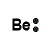
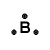
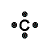
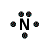

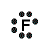

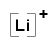
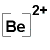
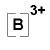 OR B3+
OR B3+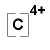
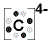
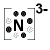
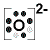
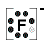
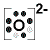 Li+ OR
Li+ OR 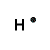
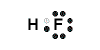 OR
OR 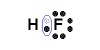
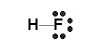
 OR
OR 

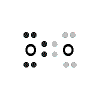 OR
OR